2207
Lower Cerebral Venous Oxygenation in Post-acute Sequelae of COVID-191Department of Diagnostic Radiology & Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, United States, 2Department of Radiology, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3Program in Neuroscience, University of Maryland School of Medicine, Baltimore, MD, United States, 4Department of Medicine, University of Maryland School of Medicine, Baltimore, MD, United States
Synopsis
Post-COVID Syndrome (PCS) is highly prevalent amongst COVID-19 survivors. Neuropsychiatric symptoms are often reported in patients with PCS, suggesting brain involvement in the early stages of COVID-19. One potential etiology is the cerebral microcirculation dysfunction due to SARS-CoV-2 infection, which may affect oxygen delivery and consumption in the brain. In the present study, we investigated the changes in cerebral venous oxygenation, which reflects the balance between oxygen supply and consumption, in convalescent COVID-19 participants with PCS. Our results showed that participants with PCS had altered venous oxygenation in their brain, which was also associated with slower locomotion.
Introduction
Post-COVID Syndrome (PCS) is highly prevalent (~50-70%) amongst COVID-19 survivors, and may last one year or longer after the acute infection [1, 2]. Neuropsychiatric symptoms are often reported in patients with PCS, and can be present even in those with mild COVID-19 symptoms [1-3], suggesting brain involvement in the early stages of COVID-19. However, despite the high prevalence of PCS, little is known regarding its etiology or the neuropathophysiology. One potential etiology is the dysfunction of cerebral microcirculation due to SARS-CoV-2 infection [4], which may affect oxygen delivery and consumption in the brain. Cerebral venous oxygenation (Yv) is an important hemodynamic parameter reflecting the balance between oxygen supply and consumption, and is a sensitive biomarker of brain function and tissue viability in several diseases [5, 6]. In the present study, we aimed to investigate Yv changes associated with PCS. To achieve this goal, we utilized a T2-relaxation-under-spin-tagging (TRUST) MRI technique to assess Yv in participants with PCS and compared it to that measured in healthy controls. Possible correlations between Yv and neurobehavioral measures were also evaluated.Methods
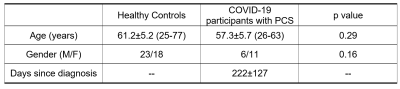
Experiment: 17 participants with PCS and 41 age-matched healthy controls were studied. Demographic information of all subjects are shown in Table 1. The PCS participants were convalescents from COVID-19 for at least 6 weeks and still have symptoms. All 17 PCS participants and 14 uninfected controls were also evaluated with the NIH-Toolbox® (NIHTB), including the Cognition Battery, Emotional Battery and Motor Battery.Assessment of Yv: Global Yv was measured by the established TRUST MRI technique [7-9] using Siemens 3T scanners. The TRUST technique uses the spin-label principle on the venous side to separate pure venous blood and measure its T2, and then convert T2 to Yv using a calibration plot based on the well-known relationship between T2 and Yv. Imaging parameters were: voxel size 3.44x3.44x5mm3, TR=3000ms, TI=1022ms, four eTEs: 0, 40, 80 and 160ms, labeling thickness 100mm, gap 22.5mm, scan duration 1.2min. The imaging slice was positioned parallel to the anterior-commissure posterior-commissure line at 20mm above the sinus confluence. The TRUST data were processed following the procedure described previously[8]. Briefly, after pairwise subtraction between control and label images, a preliminary region of interest (ROI) was manually drawn to include the superior sagittal sinus. Then, 4 peak voxels in the ROI were chosen as the final mask for spatial averaging. The averaged venous blood signals were used to fit a mono-exponential model to obtain T2, which was in turn converted to Yv via a calibration plot [9].
Statistical analysis: Yv values were compared between the two groups using a two-sample t-test. Regression analysis was also performed in all subjects with Yv as dependent variable, and group, age and sex as independent variables. Within the 17 participants with PCS, regression analyses were performed to study the correlations between cognitive scores and Yv.
Results
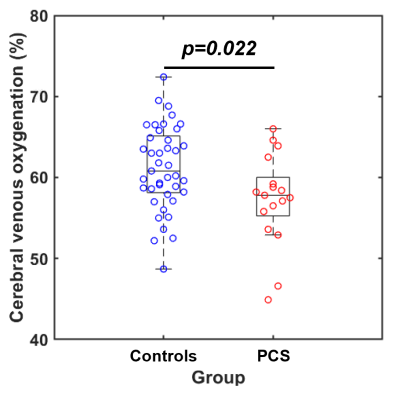
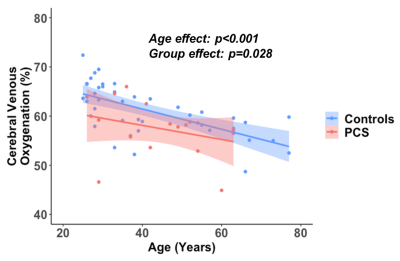
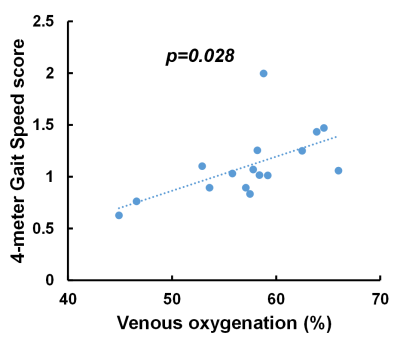
Figure 1 shows the comparison between the PCS group and uninfected-control group. Participants with PCS had significantly lower Yv compared to uninfected controls. Figure 2 shows the scatter plot of Yv as a function of age among the two groups. In both groups, Yv decreases with age, which is consistent with previous report, but the PCS group showed lower Yv across the entire age range. Regression analysis revealed significant effects of age (p<0.001) and group (p=0.024) on Yv. However, no sex-effect (p=0.78) or interaction between age and group (p=0.51) were observed. We did not observe group differences on the NIHTB-Cognitive Battery. However, on the NIHTB-Motor Battery, the PCS participants were slower on dexterity (Pegboard dominant hand, p=0.01), the 4-meter walk gait speed and had poorer endurance (2-minute walk, p=0.0015).Finally, within the PCS group, after regressing out the age effect, Yv was found to be significantly associated with the 4-meter walk gait speed score, a measure of locomotor function. As shown in Figure 3, participants with PCS who had lower Yv tended to have slower gait speed (p=0.028).
Discussion
Mounting evidence suggests that dysfunction in cerebral microcirculation plays a major role in the pathogenesis of neuropsychiatric complications in COVID-19 patients with PCS. SARS-CoV-2 can enter the brain via binding of its spike protein to the angiotensin-converting enzyme (ACE) 2 receptors, which are widely located, especially on the vascular endothelial cells [4]. Damage to the endothelia in the brain vasculature may result in diminished cerebral perfusion, and thus, insufficient oxygen supply to the brain, which in turn may cause chronic hypoxia. The relatively lower global Yv in our COVID-19 participants with PCS supports our hypothesis that brain hypoxia might contribute to symptoms associated with PCS. Interestingly, lower Yv in COVID-19 participants was associated with slower locomotion. Our findings demonstrate the sensitivity of venous oxygenation as a potential biomarker in patients with PCS. Ongoing analyses will further evaluate the relationships between psychiatric symptoms and Yv. Longitudinal follow-up studies are needed to evaluate whether the neuropsychiatric symptoms might resolve and whether the lower Yv will normalize in patients with PCS.Conclusion
Participants with PCS had altered venous oxygenation in their brain, which was also associated with slower locomotion. Therefore, cerebral venous oxygenation is a promising biomarker to evaluate PCS in future studies.Acknowledgements
No acknowledgement found.References
1. Moreno-Perez, O., et al., Post-acute COVID-19 syndrome. Incidence and risk factors: A Mediterranean cohort study. J Infect, 2021. 82(3): p. 378-383.
2. Maestre-Muniz, M.M., et al., Long-Term Outcomes of Patients with Coronavirus Disease 2019 at One Year after Hospital Discharge. J Clin Med, 2021. 10(13).
3. Heneka, M.T., et al., Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alzheimers Res Ther, 2020. 12(1): p. 69.
4. Hamming, I., et al., Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol, 2004. 203(2): p. 631-7.
5. Ge, Y., et al., Characterizing brain oxygen metabolism in patients with multiple sclerosis with T2-relaxation-under-spin-tagging MRI. J Cereb Blood Flow Metab, 2012. 32(3): p. 403-12.
6. Jiang, D., et al., Brain Oxygen Extraction Is Differentially Altered by Alzheimer's and Vascular Diseases. J Magn Reson Imaging, 2020. 52(6): p. 1829-1837.
7. Lu, H. and Y. Ge, Quantitative evaluation of oxygenation in venous vessels using T2-Relaxation-Under-Spin-Tagging MRI. Magn Reson Med, 2008. 60(2): p. 357-63.
8. Liu, P., F. Xu, and H. Lu, Test-retest reproducibility of a rapid method to measure brain oxygen metabolism. Magn Reson Med, 2013. 69(3): p. 675-81.
9. Lu, H., et al., Calibration and validation of TRUST MRI for the estimation of cerebral blood oxygenation. Magn Reson Med, 2012. 67(1): p. 42-9.
Figures