2201
Brain GABA and glutathione measurements in convalescent COVID-19 subjects1Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, United States, 2Department of Neurology, University of Maryland School of Medicine, Baltimore, MD, United States, 3Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 4Institute of Human Virology, University of Maryland School of Medicine, Baltimore, MD, United States, 5Department of Medicine, University of Maryland School of Medicine, Baltimore, MD, United States
Synopsis
COVID-19 infection was associated with lower plasma levels of glutathione (GSH), provoking oxidative stress; however, whether GSH levels are also lower in the brain is unknown. COVID-19 survivors (COVID+) also have higher prevalence of psychiatric disorders, possibly due to altered brain GABA. However, brain GABA and GSH levels have not been assessed in convalescent COVID-19 (COVID+) individuals. Using edited MRS, frontal grey matter (FGM) GSH were lower in male COVID+, and COVID+ individuals had greater age-related decline in FGM GSH, compared to uninfected controls. These findings suggest that male COVID+ individuals, especially the older subjects, are susceptible to oxidative stress.
Introduction
The Coronavirus Disease of 2019 (COVID-19) has spread globally and can cause severe illness and death in some individuals. The severity of COVID-19 infection is associated with excessive levels of plasma reactive oxygen species (ROS) and higher plasma ratios of ROS to the antioxidant glutathione (GSH), provoking oxidative stress.1-3 However, plasma levels may not reflect the brain levels of GSH. COVID-19 survivors also have a higher prevalence of psychiatric diseases,4 which are often related to altered brain levels of γ-aminobutyric acid (GABA).5 In this study, we use edited magnetic resonance spectroscopy (MRS)6, 7 to measure in vivo brain levels of GSH and GABA. We hypothesized that GSH and GABA levels will be abnormal in convalescent COVID-19.Methods
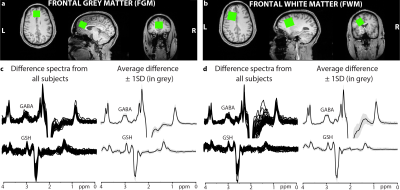
Participants: 17 uninfected controls (9 men/8 women; age: 44.0±12.7 years) and 21 COVID-19 subjects (7 men/14 women; age: 45.8±13.8 years, 186±100 days since COVID-19 diagnosis).MRI/MRS protocol: Siemens 3T Prisma scanner with a 64-channel head coil was used to acquire a whole-brain 1 mm3 MPRAGE image to guide voxel placements in the frontal grey matter (FGM) and frontal white matter (FWM) (Figures 1a-b). Parameters for the edited MRS were: voxel size 18.75 ml; TE/TR 80/2000 ms; 20-ms editing pulse duration; 4096 datapoints; 4-kHz spectral width; and 320 transients. Data were acquired using the HERMES sequence6,7 for the simultaneous editing and measurement of GABA (GABA and macromolecules) and GSH followed by unsuppressed water acquisition.
Data Processing: Data were processed in Gannet.8 The GABA signal (GABA and macromolecules) at 3 ppm, GSH at 2.95 ppm, and the water signal were modeled to generate absolute concentrations in institutional units. MPRAGE images were segmented to calculate grey matter, white matter, and cerebrospinal fluid voxel tissue fractions, which in turn were used to correct the concentrations for tissue-dependent signal attenuation.9 Data quality was assessed using magnetic field (B0) drift and NAA signal linewidth at full-width half-maximum. Also, the GABA and GSH fit errors (ratio of the standard deviation of the fit residual to the amplitude of the modeled peak) and model linewidth from Gannet were used for assessing modeling errors. The average and standard deviation (SD) of all fit and modelling errors (separately for each metabolite and region) were calculated across subjects. Data with values outside of the mean ±3SDs were rejected for statistical analysis.
Analysis: Two-way analyses of covariance (ANCOVA) were performed to evaluate the effects of group status (COVID+ or controls), age, and their interactions (group status x age) on GABA and GSH concentrations. Sex-specific differences in concentrations were evaluated. A p-value < 0.05 were considered significant.
Results
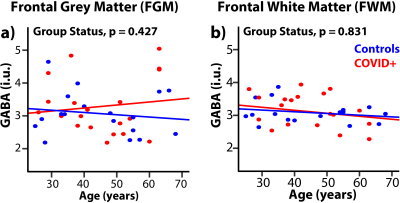
Figures 1c-d show GABA- and GSH-edited spectra from all subjects and average spectra from the FGM and FWM region. B0 drift (and linewidth) from the 10-min acquisition was 1.0±1.3 Hz (8.9±1.0 Hz) in the FGM and 1.9±1.2 Hz (7.9±1.0 Hz) in the FWM, indicating excellent frequency stability and B0 homogeneity. One GSH-edited spectrum from FGM and two GABA-edited spectra from FWM were removed due to fitting or modelling errors exceeding the threshold.The FGM GSH showed a trend for lower concentrations in COVID+ than control subjects (–12%, ANCOVA-p=0.063, Figure 2a), which was due to lower GSH in COVID+ men (–22%, p=0.039) compared to uninfected men (Figure 2b). The COVID+ group also showed an age-related decline in GSH but not in the controls (interaction-p=0.039, Figure 2a). Hence, older COVID+ subjects had lower GSH with increasing age (r=–0.53, p=0.015). However, the FWM GSH showed mildly elevated GSH in COVID+ subjects compared to controls across the age range (+14%, p=0.21, Figure 2c). The FGM and FWM GABA concentrations were not different between COVID+ and control subjects (Figure 3).
Discussion
To our knowledge, this is the first study to employ edited MRS to measure brain GABA and GSH levels non-invasively in convalescent COVID subjects. We found lower GSH levels in the COVID+ men, as well as in the older participants, which suggest that they had lower antioxidant capacity. In contrast, the GSH levels were mildly elevated in the FWM of COVID+ subjects, which suggests a compensatory response to oxidative stress. Oxidative stress results from an increased generation of ROS (free radicals) and reduced antioxidant defense. Higher levels of ROS may impair adaptive immunity, vulnerability to viral infections, induction of oxidative damage of endogenous molecules, such as proteins and DNA, that lead to cell damage.10 Since higher levels of GSH levels are essential to reduce ROS in the brain, exogenous antioxidants (oral supplements11) might be considered in the therapeutic strategy for patients with COVID-19. Although GABA abnormalities are often reported in patients with psychiatric disorders, we did not observe them in our COVID+ participants. Since the current study only evaluated two brain regions and had a relatively small sample size, future studies should use a larger sample size to investigate GABA and GSH levels across more brain regions. Longitudinal studies are also warranted to determine whether the lower GSH levels might normalize with a longer duration of recovery.Acknowledgements
This work was supported by NIH grants R21NS121615 and K99DA051315.References
1. Fu, J., et al., The clinical implication of dynamic neutrophil to lymphocyte ratio and D-dimer in COVID-19: A retrospective study in Suzhou China. Thrombosis research, 2020. 192: p. 3-8.
2. Polonikov, A., Endogenous deficiency of glutathione as the most likely cause of serious manifestations and death in COVID-19 patients. ACS infectious diseases, 2020. 6(7): p. 1558-1562.
3. Pincemail, J., et al., Oxidative stress status in COVID-19 patients hospitalized in intensive care unit for severe pneumonia. A pilot study. Antioxidants, 2021. 10(2): p. 257.
4. Taquet, M., et al., Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. The Lancet Psychiatry, 2021. 8(2): p. 130-140.
5. Schmidt-Wilcke, T., et al., GABA—from inhibition to cognition: emerging concepts. The Neuroscientist, 2018. 24(5): p. 501-515.
6. Saleh, M.G., et al., Simultaneous edited MRS of GABA and glutathione. NeuroImage, 2016. 15: p. 576-582.
7. Saleh, M.G., et al., Multi-vendor standardized sequence for edited magnetic resonance spectroscopy. NeuroImage, 2019. 189: p. 425-431.
8. Edden, R.A., et al., Gannet: A batch‐processing tool for the quantitative analysis of gamma‐aminobutyric acid–edited MR spectroscopy spectra. Journal of Magnetic Resonance Imaging, 2014. 40(6): p. 1445-1452.
9. Gasparovic, C., et al., Use of tissue water as a concentration reference for proton spectroscopic imaging. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 2006. 55(6): p. 1219-1226.
10. Bakadia, B.M., et al., The impact of oxidative stress damage induced by the environmental stressors on COVID-19. Life sciences, 2021. 264: p. 118653.
11. Poe, F.L. and J. Corn, N-Acetylcysteine: A potential therapeutic agent for SARS-CoV-2. Medical hypotheses, 2020. 143: p. 109862.
Figures