2198
Population-receptive field size estimates are biased by acquisition and preprocessing parameters: a 7T fMRI study1High Field MR Center, Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, Wien, Austria, 2Department of Ophtalmology, Medical University of Vienna, Wien, Austria
Synopsis
Population receptive field (pRF) mapping is an advanced retinotopic mapping approach. While the size of pRFs has been identified as an important neurophysiological feature, remarkable differences in the pRF sizes across studies has been reported. In this study, we examine the effects of difference in spatial resolution on the pRF size obtained in a group of ten healthy subjects at 7T. Our results clearly show that the amount of spatial dependence between voxels in the visual cortex has a direct effect for the pRF sizes calculated. We can assume similar effects in data sets acquired at different spatial resolutions.
INTRODUCTION
Functional magnetic resonance imaging is an ideal non-invasive method for examining the features of the visual system. In particular, the retinotopic organisation, i.e. the fact that information in a given part of the visual is processed by a specific area on the visual cortex, has been studied extensively with fMRI. Early studies have used travelling wave paradigms like rotating wedges of expanding rings to map this retinotopic structure, resulting in eccentricity and polar angle values for each voxel within the visual cortex1. Population receptive field (pRF) mapping is an advanced retinotopic mapping approach, where pRF sizes can be estimated as additional parameter2. This pRF size corresponds to the receptive field within the visual field of view of a particular voxel on the visual cortex. In pRF mapping, different to the travelling wave approach, arbitrary stimulus configuration can be used. Retinotopic parameters are estimated by fitting 2D isotropic Gaussian models calculated from the visual stimuli employed. While the size of pRFs has been identified as an important neurophysiological feature of the visual system, remarkable differences in the pRF sizes across studies has been reported3-6. In this study, we examine the effects of difference in spatial resolution on the pRF size obtained.METHODS
Ten subjects (6 male, 4 female; age 25.0±2.8) participated in this study. Only subjects with a refractive error of less than 6 diopters and without significant ocular disease, history of trauma or eye surgery were included. They were naive to the experiment, were introduced to the stimuli only shortly before the measurement and received no further training. Subjects gave informed written consent and received financial compensation for their participation. Measurements were performed on an ultra-high field 7 Tesla MAGNETOM scanner (Siemens Healthineers, Erlangen, Germany) using a 32-channel head coil. Functional data were acquired using the CMRR EPI sequence (Moeller et al., 2010) measuring 32 slices with 1 mm isotropic resolution and the following parameters: TE=25 ms, TR=1000 ms, multiband factor=2, GRAPPA acceleration=2, slice spacing=10 %. Independent of the stimulation paradigm used, 336 volumes were acquired per run, corresponding to a run time of about 5.5 minutes. Two runs were acquired in each subject. Slices were positioned orthogonally to the calcarine sulcus, covering 35.2 mm of the occipital cortex. Additionally, B0 field maps were acquired for distortion correction. Anatomical imaging was performed using a magnetization-prepared rapid gradient-echo (MPRAGE) sequence with 0.7 mm isotropic resolution (TE=3.66 ms; TR=1960 ms). Bar/ring stimuli were used which each covered the central 14° of the subjects' visual field. Subjects were instructed to fixate a central dot and report colour changes to ensure fixation and quantify the subjects' attention during the task. All stimuli are shapes exposing an 8 Hz black-white reversing checkerboard. Preprocessing of functional data included slice-timing correction using SPM12 (https://www.fil.ion.ucl.ac.uk/spm) in Matlab 9.6, as well as realignment (SPM) and distortion correction using the acquired field maps and FSL FUGUE. For obtaining cortical grey matter masks, segmentation using the Freesurfer image analysis suite (https://surfer.nmr.mgh.harvard.edu) was applied to the high-resolution MPRAGE anatomical image. Matlab toolbox mrVista (https://web.stanford.edu/group/vista/CGI-bin/wiki/index.php/MrVista) was used for generating stimuli and analyzing pRF data. Run-length for each stimulus variant was 336s. We used spatial smoothing as proxy for changes in the spatial resolution. Applying 3D Gaussian smoothing with FWHM of 2, 4, and 8 mm, we thus obtained three addition data sets per subject and used the identical preprocessing and analysis pipeline as for the unsmoothed fMRI data.RESULTS
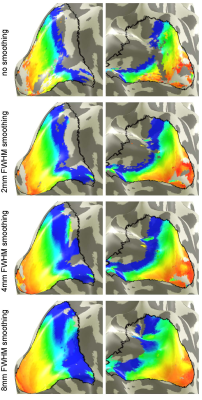
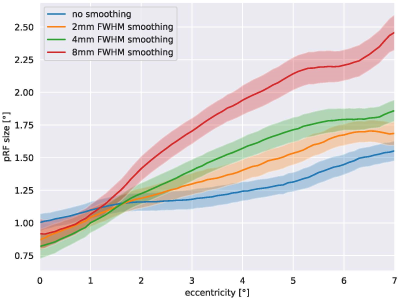
Figure 1 shows eccentricity maps overlaid to the cortical surface of one representative subjects. All maps follow the expected layout. Spatial smoothing increases SNR and thus the number of supra-threshold voxels increases as well. Fig. 2 plots the group-averaged pRF sizes over eccentricity for the different smoothing kernels. It can be seen clearly that higher smoothing kernel sizes lead to increased pRF sizes.DISCUSSION
Our results clearly show that the amount of spatial dependence between voxels in the visual cortex has a direct effect for the pRF sizes calculated. This is an important finding as it demonstrates that cross-study comparisons of pRF size estimates need to include the degree of smoothing used in the data preprocessing pipeline. In addition, we can assume that similar effects will be present in data sets acquired at different spatial resolutions.CONCLUSION
Population receptive field sizes as estimated by fMRI retinotopic mapping depend on the acquisition and preprocessing parameters used. Additional research is needed to develop methods for reducing this bias in order to obtain the underlying, neurophysiologically valid pRF sizes.Acknowledgements
This work was supported by the Austrian Science Fund (FWF) [grant numbers: KLI 670; P 33180]. All authors declare no financial interests or potential conflicts of interest in relation to the work described.
References
1: Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RB. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995 May 12;268(5212):889-93. doi: 10.1126/science.7754376. PMID: 7754376.2: Wandell BA, Winawer J. Computational neuroimaging and population receptive fields. Trends Cogn Sci. 2015 Jun;19(6):349-57. doi: 10.1016/j.tics.2015.03.009. Epub 2015 Apr 4. PMID: 25850730; PMCID: PMC4484758.
3: Himmelberg MM, Kurzawski JW, Benson NC, Pelli DG, Carrasco M, Winawer J. Cross-dataset reproducibility of human retinotopic maps. Neuroimage. 2021 Dec 1;244:118609. doi: 10.1016/j.neuroimage.2021.118609. Epub 2021 Sep 25. PMID: 34582948; PMCID: PMC8560578.
4: Amano K, Wandell BA, Dumoulin SO. Visual field maps, population receptive field sizes, and visual field coverage in the human MT+ complex. J Neurophysiol. 2009 Nov;102(5):2704-18. doi: 10.1152/jn.00102.2009. Epub 2009 Jul 8. PMID: 19587323; PMCID: PMC2777836.
5: Barbot A, Das A, Melnick MD, Cavanaugh MR, Merriam EP, Heeger DJ, Huxlin KR. Spared perilesional V1 activity underlies training-induced recovery of luminance detection sensitivity in cortically-blind patients. Nat Commun. 2021 Oct 20;12(1):6102. doi: 10.1038/s41467-021-26345-1. PMID: 34671032; PMCID: PMC8528839.
6: Alvarez I, Hurley SA, Parker AJ, Bridge H. Human primary visual cortex shows larger population receptive fields for binocular disparity-defined stimuli. Brain Struct Funct. 2021 Dec;226(9):2819-2838. doi: 10.1007/s00429-021-02351-3. Epub 2021 Aug 4. PMID: 34347164; PMCID: PMC8541985.