2194
CBV-sensitive layer-fMRI in the human auditory cortex at 7T: Challenges and capabilities1Department of Cognitive Neuroscience, Faculty of Psychology and Neuroscience, Maastricht University, Maastricht, Netherlands, 2Brain Innovation, Maastricht, Netherlands
Synopsis
Laminar-specific fMRI with CBV-sensitive VASO is valuable for neuroscientific questions on hierarchical information processing. While VASO fMRI has already proven its utility in other brain areas, it has not yet been successfully applied in the auditory cortex due to several additional technical challenges in this region. Here, we explore multiple sequences and their effectiveness to mitigate these challenges. Our purpose is to develop an experimental setup that future neuroscientific studies can be built on. Ultimately, we found a stable parameter set for the usage of layer-fMRI VASO in the auditory cortex and validated it in a group of participants.
Introduction
Laminar-specific fMRI with blood volume sensitive VASO methods allows neuroscientists to address research questions of hierarchical information processing across brain areas without unwanted large draining vein effects. While layer-fMRI VASO has been used for visual and sensory-motor areas before, its application in the auditory system is challenged by many sequence constraints (Fig. 1):- Inflow contaminations by non-nulled blood;
- Limited B1+-efficiency for the inversion of z-magnetization;
- Physiological noise variation across segments in the common VASO 3D-EPI acquisitions;
- Evolution of non-steady-state magnetization in inversion recovery sequences with silent gaps;
- Generally low sensitivity compared to GE-BOLD, due to the lack of signal amplification at the large draining veins.
- 2D-EPI, 3D-EPI1, and MB-EPI-VASO2;
- Multi-shot and single-shot schemes for GRAPPA auto-calibration signal data3,4;
- Multiple orientations (coronal vs. sagittal) and timing (faster and slower than cardiac cycle);
- Advanced adiabatic inversion pulses with B1+-insensitive adjustable inversion efficiency5 and non-adiabatic Mz-’rest’ pulses6.
Methods
Scanning was performed on a MAGNETOM “classic” 7T scanner (Siemens Healthineers) with MRI-compatible earbuds (Sensimetrics) for presentations of sounds in the scanner. For simultaneous acquisition of BOLD and VASO, an SS-SI-VASO sequence was used7,8. A total of 21.5 hours of scanning was done:- Six hours of phantom scanning was done to validate the correct implementation of the above mentioned sequence approaches.
- Seven and a half hours of in-vivo scanning across 4 sessions was performed to find optimal parameters (Fig. 1).
- Four two-hour sessions were performed to validate the stability of the chosen sequence approach (Fig. 2) in experienced and in naive participants.
Data processing: Motion corrected (SPM) data were sorted by contrast and corrected for BOLD contamination (LayNii). Equi-volume layerification was performed with LN2_LAYERS9. Block design activation z-scores were quantified with FSL Feat and are presented as an overlay to the inherently T1-weighted mean VASO images (Fig. 3-4).
Results and Discussion
During the first ten hours of scanning, we found that each and every one of the challenges in layer-fMRI VASO at the auditory cortex can be partially accounted for by means of advanced sequence approaches and field of view compromises. We tested the reproducibility of our findings with 2D and 3D acquisition protocols of BOLD and VASO by applying the identical sequence and task settings in the same volunteers in four additional two-hour sessions. We find that both acquisition approaches (2D and 3D) and contrasts (VASO and BOLD) reveal significant activation in the primary auditory cortex (Fig. 3). Layer-profiles of VASO have the largest activation in slightly deeper cortical depths compared to BOLD. In the BOLD data, the signal increases towards the superficial surface. The results are consistent across sessions and participants (Fig. 4).Summary and Conclusion
While layer-fMRI VASO has become popular in many cortical areas, until now it has never been successfully applied in the human auditory cortex. This is in contrast to GE-BOLD readouts, which have been applied in the auditory cortex with success already10,11. Here, we described why VASO layer-fMRI is so challenging in this area and we present a combination of sequence approaches as well as a good parameter set for successful layer-fMRI VASO without venous biases in the auditory cortex. This study has built the groundwork that future neuroscience-focused application studies can be based on.Acknowledgements
Help with scanning: These data were acquired with the kind support of Scannexus. We thank Miriam Heynckes for advice on the use of auditory stimulation setups. We thank Chris Wiggins for providing the 3rd order shimming tools used here.
Funding: Scanning was supported by FPN (Faculty of Psychology and Neuroscience) via the MBIC grant scheme. LKF was funded by the National Institute for Health grant RF1MH116978-01. OFG is an employee of Brain Innovation and has financial interests tied to the company. FDM was funded by the National Institute for Health grant RF1MH116978-01 and the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No. 101001270. BAP is funded by the NWO VIDI grant 16.Vidi.178.052, the National Institute for Health grant R01MH/111444 (PI David Feinberg) and by the H2020 FET-Open AROMA grant agreement no. 88587. RH was funded by the NWO VENI project 016.Veni.198.032.
Ethics: The scanning procedures have been approved by the Ethics Review Committee for Psychology and Neuroscience (ERCPN) at Maastricht University, following the principles expressed in the Declaration of Helsinki.
References
1. Poser BA, Koopmans PJ, Witzel T, Wald LL, Barth M. Three dimensional echo-planar imaging at 7 tesla. Neuroimage. 2010;51(1):261-266. doi:10.1016/j.neuroimage.2010.01.108
2. Huber L, Ivanov D, Guidi M, et al. Functional cerebral blood volume mapping with simultaneous multi-slice acquisition. Neuroimage. 2016;125:1159-1168. doi:10.1016/j.neuroimage.2015.10.082
3. Polimeni JR, Bhat H, Witzel T, et al. Reducing sensitivity losses due to respiration and motion in accelerated echo planar imaging by reordering the autocalibration data acquisition. Magn Reson Med. 2016;75(2):665-679. doi:10.1002/mrm.25628
4. Talagala SL, Sarlls JE, Liu S, Inati SJ. Improvement of temporal signal-to-noise ratio of GRAPPA accelerated echo planar imaging using a FLASH based calibration scan. Magn Reson Med. 2016;75(6):2362-2371. doi:10.1002/mrm.25846
5. Norris DG. Adiabatic radiofrequency pulse forms in biomedical nuclear magnetic resonance. Concepts Magn Reson Part B Magn Reson Eng. 2002;14(2):89-101. doi:10.1002/cmr.10007
6. Lu H. Magnetization “reset” for non-steady-state blood spins in Vascular-Space-Occupancy (VASO) fMRI. Proc 16th Annu Meet ISMRM. 2008;16(1):2008. http://cds.ismrm.org/ismrm-2008/files/00406.pdf
7. Lu H, Golay X, Pekar JJ, van Zijl PCM. Functional magnetic resonance imaging based on changes in vascular space occupancy. Magn Reson Med. 2003;50:263-274. doi:10.1002/mrm.10519
8. Huber L, Ivanov I, Krieger SN, Streicher MN, Mildner T, Poser BA, Moller HE, Turner R. Slab‐selective, BOLD‐corrected VASO at 7 Tesla provides measures of cerebral blood volume reactivity with high signal‐to‐noise ratio. Magnetic resonance in medicine, 2014;72.1: 137-148.
9. Huber L, Poser BA, Bandettini PA, et al. LAYNII: A software suite for layer-fMRI. Neuroimage. 2021;237:118091. doi:10.1016/j.neuroimage.2021.118091
10. De Martino F, Moerel M, Ugurbil K, Goebel R, Yacoub E, Formisano E. Frequency preference and attention effects across cortical depths in the human primary auditory cortex. Proc Natl Acad Sci. 2015;112(52):16036-16041. doi:10.1073/pnas.1507552112
11. Moerel M, De Martino F, Kemper VG, et al. Sensitivity and specificity considerations for fMRI encoding, decoding, and mapping of auditory cortex at ultra-high field. Neuroimage. 2018;164(March):18-31. doi:10.1016/j.neuroimage.2017.03.063
Figures
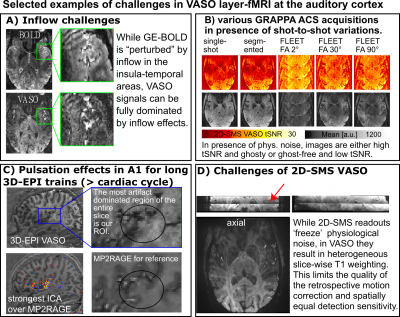
Figure 1: Selected challenges of performing layer-fMRI VASO in the auditory cortex at 7T
The blood vessels close to the auditory cortex are challenging on so many levels. A) They result in dominating inflow contaminations, B) they impose physiological noise within the GRAPPA auto calibration signal, C) they evoke signal changes that are in the order of magnitude as structural and functional VASO contrast, D) common approaches that would minimize these effects impose other VASO-related problems.
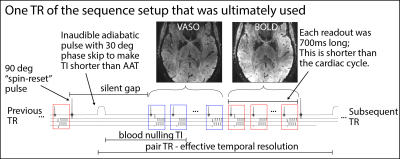
Figure 2: Schematic sequence diagram and the adaptation for VASO application in the auditory cortex at 7T
The sequence setup that was found to be most suitable is an axial acquisition with spin-reset pulses at the end of each readout. A TR-FOCI pulse with phase skip was used for efficient inversion of a single channel head-Tx NOVA coil. Readout blocks were kept as short as possible at the cost of coverage. The inherent silent sequence dead times in VASO were exploited for auditory task presentation.
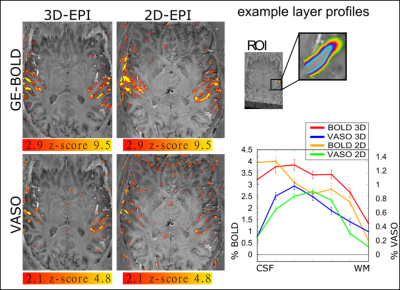
Figure 3: Representative activation results Activation maps of GE-BOLD and VASO for 3D-EPI and 2D-EPI (no SMS)
We find that the fast protocol with readout durations of 700ms and a thin slab with 12 slices at 0.9mm iso can detect significant activation changes. For BOLD, z-scores are generally larger with 2D-EPI in the activation maps, while for VASO, z-scores are larger for 3D-EPI. As expected, layer-profiles for GE-BOLD are slightly shifted towards the surface.
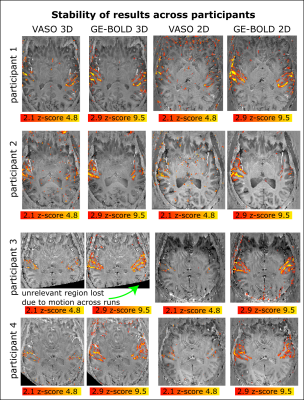
Figure 4: Stability of VASO activation across 4 participants
It can be seen that the sequence approaches investigated here provide enough sensitivity to provide layer-fMRI results in the auditory cortex. Two of the participants were naive to fMRI scanning and two of them were experienced fMRI participants. There is an indication that 3D-EPI provides more activation for VASO than 2D-EPI. And 2D-EPI provides more activation for BOLD than 3D-EPI. VASO is less sensitive than BOLD.