2192
Using ultra-high field fMRI to uncover depth-dependent activation in the ventral temporal lobe1Neurosurgery, Center for Magnetic Resonance Research, Minneapolis, MN, United States, 2Neurosciences, Center for Magnetic Resonance Research, Minneapolis, MN, United States, 3Center for Magnetic Resonance Research, Minneapolis, MN, United States
Synopsis
We used 7T BOLD functional imaging to acquire 0.7mm voxels and the thermal noise suppression technique NORDIC to evaluate depth-dependent, task related top-down modulations. Four participants viewed face stimuli and performed either a face or fixation task (identical stimuli). We compared depth-dependent task modulations in the primary visual cortex (V1) and fusiform face area (FFA). We found that, relative to the fixation task, face detection led to larger modulations in outer relative to inner layers in the FFA, and the inverse pattern in V1. This is a critical step towards using laminar analyses to examine hierarchical or distributed cognitive processes.
Introduction
Over the last 25 years, functional MRI (fMRI) has become one of the main tools for human neuroscience. The high spatial resolution and non-invasiveness of fMRI uniquely position it as a tool for the study of human brain function, including high level cognitive processes. Advances in both hardware and software have led to the acquisition of functional images with unprecedented spatial resolution, spanning the submillimeter range. These high-resolution measurements permit the study of human mesoscale functional organization, that is (i.e., layers and columns). To balance current imaging acquisitions capabilities against signal-to-noise constraints, many of these studies are carried out using 0.8mm isotropic voxels, which, although partially successful, may be insufficient to fully characterize laminar functional profiles. Until recently, the details of layers and columns, which represent a scale of organization for some of the most fundamental units of neural computations1, could only be studied using invasive mechanisms in animal models. Understanding this fine scale organization may be essential to understanding high level cognitive processes that are unique to humans. The need to keep pushing imaging resolution is therefore self-evident. This endeavor, while in principle achievable with highly accelerated acquisition protocols, is limited by the deleterious impact of thermal noise. Here we used 7 Tesla functional neuroimaging to acquire 0.7mm isotropic voxels, achieving a voxel volume of 0.343 μL relative to the 0.512 μL obtained with 0.8mm acquisitions (a 33% reduction). Importantly, we processed this data with our recently proposed denoising algorithm, NORDIC, that effectively suppresses thermal noise without impacting the spatial precision of functional measurements2 . We then evaluated the top-down effects of high level and socially relevant task demands, such as face perception, across cortical depths in lower and higher-level visual areas using a face processing paradigm. We have shown, with a similar paradigm using supramillimeter fMRI, that task demands significantly modulate top-down responses to identical visual stimuli3 - here we aimed to elucidate the laminar basis of these modulations.Methods
Four participants (3 male, 1 female) completed a 7T scanning session imaging the occipital pole through mid-ventral temporal cortex (Gradient echo EPI; TR: 2.030s; R=3; MB=2; 6/8ths Partial Fourier; TE: 29.6ms; 56 slices). In two tasks, subjects viewed 8 blocks (12s on/off duration) of face stimuli (run duration: 3:33). Faces were partially scrambled between 0 to 40% phase coherence (10% steps) (Figure 1A). Participants completed 6 runs of a face detection task, in which they indicated if they perceived a face, and 6 runs of a fixation task, in which they indicated when the fixation cross turned red (Figure 1B). Before standard preprocessing (i.e., slice-timing; motion and detrending) images were processed using NORDIC (Figure 2). Reverse phase images were used to distort T1 (0.75 mm iso MP2RAGE4) anatomical images into functional space. Regions of interest (ROIs) were drawn constrained to the gray matter, using a separate face localizer (Figure 3A) (Occipital face area: OFA; fusiform face area: FFA) or the first block of each task (primary visual cortex: V1), which was discarded for subsequent analysis. The regions of interest were then converted into 3 layers (inner, middle, outer, Figure 3B) using the LayNii toolbox5. Activation was estimated for each single-trial (i.e., a block). To evaluate top-down effects of task demands, we calculated the ratio of the activation during the face task relative to the fixation task for each layer.Results
As expected, regardless of task and ROI, we report a typical vessel related increase in the magnitude of the BOLD responses towards the pial surface, as is observed with gradient echo EPI (Figure 4). We show however that, relative to the fixation task, the face task elicited larger BOLD responses in the inner compared to the outer layers of V1; and in the outer compared to the inner layers in the FFA (Figure 5), both findings significant for each subject (bootstrapped p<0.05, multiple comparison corrected).Discussion
We approached the challenge of analyzing layers in the VTC by increasing spatial resolution, improving the signal to noise ratio (SNR) with NORDIC, reducing additional blurring by remaining in distorted functional space and using well-studied task demands. Our layer dependent findings are consistent with animal reports of feedback being exchanged between deeper and superficial layers6. Furthermore, these results are consistent with a theorized mechanism of conscious perception involving apical dendritic amplification7. Importantly, the dissociation in laminar profiles between higher (i.e., FFA) and lower (i.e., V1) regions indicates that the observed results cannot be accounted for by spurious venous effects.Conclusion
NORDIC successfully reduces thermal noise, allowing further increases in the prescribed imaging resolution (i.e., 0.7mm isotropic) in challenging cortical regions, such as the human ventral cortex, while maintaining reasonable levels of SNR. Our approach found typical pial biases but more importantly distinguished layer-specific profiles of activation occurring under different tasks constraints. These activation profiles support hierarchical models of processing, representing a promising step towards characterizing laminar functional profiles in humans for complex, cognitively meaningful, and socially relevant stimuli such as faces.Acknowledgements
Funding provided by NIH Grants U01EB025144 (K.U.), P41 EB027061 (K.U.), P30 NS076408 (K.U.) and RF1 MH116978 (E.Y.), and RF1 MH117015 (G.G.)References
1. De Martino, F. et al. The impact of ultra-high field MRI on cognitive and computational neuroimaging. NeuroImage 168, 366–382 (2018).
2. Vizioli, L. et al. Lowering the thermal noise barrier in functional brain mapping with magnetic resonance imaging. Nat Commun 12, 5181 (2021).
3. Dowdle, L. T., Ghose, G., Ugurbil, K., Yacoub, E. & Vizioli, L. Clarifying the role of higher-level cortices in resolving perceptual ambiguity using ultra high field fMRI. NeuroImage 227, 117654 (2021).
4. Marques, J. P. et al. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage 49, 1271–1281 (2010).
5. Huber, L. (Renzo) et al. LayNii: A software suite for layer-fMRI. NeuroImage 118091 (2021) doi:10.1016/j.neuroimage.2021.118091.
6. Rockland, K. S. & Pandya, D. N. Laminar origins and terminations of cortical connections of the occipital lobe in the rhesus monkey. Brain Research 179, 3–20 (1979).
7. Marvan, T., Polák, M., Bachmann, T. & Phillips, W. A. Apical amplification—a cellular mechanism of conscious perception? Neuroscience of Consciousness 2021, (2021).
Figures
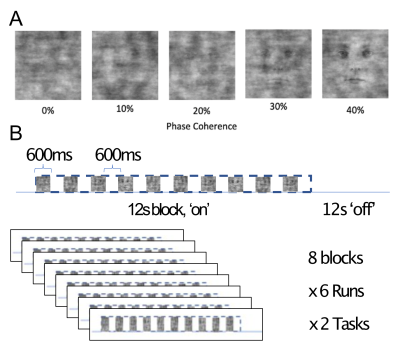
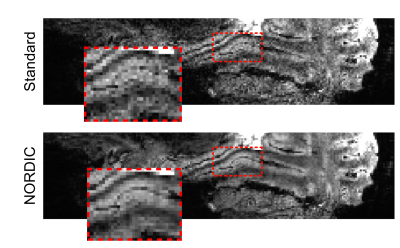
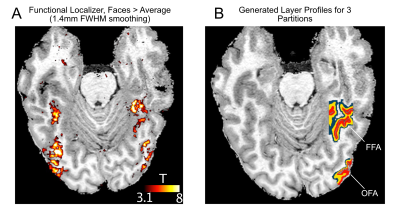
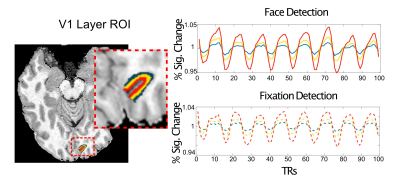
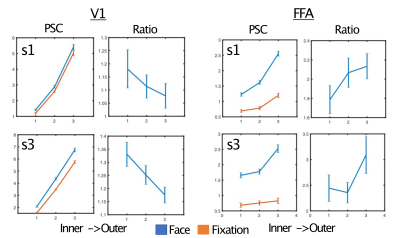
Figure 5. Dissociable task and depth dependent effects. For simplicity, two representative subjects are shown. Left) The percent signal change (PSC) and ratio of face to fixation for each layer for primary visual cortex - V1. Despite the pial bias visible in the PSC column, the ratio shows a significant decrease in outer relative to inner layers. Right) In contrast, for the FFA we instead observe a significant increase in the ratio for the outer relative to inner layers. Significance obtained by bootstrapping, with p<0.05 threshold, corrected for multiple comparisons.