2191
Laminar VASO fMRI in hand dystonia patients1HMCS, NINDS - NIH, Bethesda, MD, United States, 2Neurology, University of Utah Health, Salt Lake City, UT, United States, 3Psychology and Neuroscienc, Maastricht University, Maastricht, Netherlands
Synopsis
Mesoscopic fMRI with CBV-sensitive VASO can be a valuable tool for research questions on affected neural processing in patients suffering from neurological diseases. However, its applicability in patient populations remains unclear and is challenged by multiple methodological constraints. Here, we seek to use finger tapping tasks in hand dystonia patients to map affected topographical and laminar fMRI features. Specifically, we described the input-dominated laminar input-output circuits in the primary motor system as well as the ‘scrambled’ finger representations in the somatosensory areas. We built and validated an acquisition and analyses setup for laminar and columnar mapping in patient populations.
Purpose
Focal Hand Dystonia (FHD) is a disabling movement disorder characterized by involuntary movements, cramps and spasms.1 It is expected to come along with closer (1-2mm) and more overlapping finger representation in the somatosensory system.2-6 Sub-millimeter fMRI with blood volume sensitive VASO methods has the potential to map the alterations in the laminar microcircuitry and columnar body part representations without unwanted large draining vein effects.7,8 However, while VASO could previously capture the fine scale finger representations in healthy volunteers (HV) with 18 hours of scan data per participant, its application in FHD patients is challenging (Fig. 1):I.Head motion: Access to patients is limited. Thus, the experimenter does not have the luxury to solely invite those individuals that have the ability to lie perfectly still.
II.Noise in task compliance: FHD patients have a hard time following the task instructions. E.g. when instructed to move the ring finger only, the middle finger and pinky finger are often partly moved too. This reduces the finger functional contrast to noise ratio (CNR).
III.EPI Artifact level: Sub-millimeter fMRI is limited by low bandwidths and small coverages. For applications in HVs, this could be accounted for with iterative fine tuning of acquisition parameters (iterative B0-shimming, GRAPPA regularization, alignment, redout bandwidth). Within the limited scan time of patients, this fine tuning is not possible.
The 7T group in Nottingham has previously mastered these challenges and mapped the finger representation in FHD patients with 1.5mm GE-BOLD.9 Here we aim to use sub-millimeter vein-bias-free VASO to capture laminar and columnar digit representation in FHD patients.The purpose of the study was to use the altered digit representation in FHD patients as a test bed to explore the capability of applying layer-fMRI VASO to investigate mesoscopic body part representations in FHD patients.
Methods
Scanning was performed on a SIEMENS MAGNETOM “classic” 7T scanner while participants followed a block-designed individual finger tapping task. For layer-fMRI scanning without venous biases, a blood volume sensitive VASO7 sequence was used. Four FHD patients and four HVs (as part of previous study) were scanned. While the same tasks and sequences were executed for both patients and HVs, the HVs were invited for up to 8 additional two-hours experiments with multiple structural and functional somatosensory tasks.8 The patients, however, were ‘only’ scanned in a single two-hour session including the main four-finger tapping task set for 30 min per hand.Scanning: fMRI sequence parameters were: SS-SI-VASO,8 in-plane resolution 0.75mm, slice-thickness: 0.89mm with largely perpendicularly aligned slices. TE=24ms, 24 slices, TR/TI=2.2/1.1s, FLASH-GAPPA=3, 3D-EPI readout,10 32-ch. NOVA coil, SC72 body gradient. Complete scan parameters here: https://layerfmri.page.link/FHD_protocol
Processing: Motion corrected (SPM) data were sorted by contrast and corrected for BOLD contamination. Layerification and columnarication was done in LayNii.11 Block design activation z-scores and beta
Results and Discussion
Fig. 2 shows that the experimental sub-millimeter VASO imaging setup can reliably capture neurally-induced functional CBV changes in patients across hemispheres and individuals. Across all datasets, we find tapping induced activation both in M1 and in S1. It can be seen how the activity is confined to GM without the sensitivity of large drain pial veins above the GM surface.We imposed a local coordinate system covering the finger representations of BA3b for subsequence analysis of topographical representations of individual digits. Data of one representative participant show exemplary how the finger representation follows the somatotopic alignment for the healthy hemisphere (Fig. 3). The spatial structure of the representations in the affected hemisphere are less clear, while not being limited by detection sensitivity.
Fig. 4 depicts M1 layer-profiles of all patients. As expected, GE-BOLD signals show an increased bias towards the superficial layers compared to CBV-sensitive VASO. The affected hemispheres seem to be more dominated from superficial cortico-cortical input-layers compared to the healthy hemispheres in all participants.
Summary and Conclusion
While there are plentiful review articles about the value of layer-fMRI for clinical neuroscience research12-15 experimental layer-fMRI data are scarce.16 Here, we present fMRI results of laminar and columnar CBV changes in FHD patients.We could not confirm previous findings of closer finger representation4-6 because the finger representation in our maps did not show clear locations from which the distances could be calculated.
We find that FHD affected hemispheres had a stronger activity in motor input-layers (II/III) and reduced activity in motor output-layers (Vb/VI). This might be due to reduced surround inhibition in the superficial layers of the affected hemispheres. It could also be related to increased mental load (attention, motor planning, incorporating sensory feedback) that is terminating in the input-layers for moving the affected hand. More data are needed to ultimately determine the stability of this finding.This study has built the methodological groundwork that future clinically-focused neuroscience application studies of layer-fMRI can be based on.
Acknowledgements
Experimental setup: We thank the kind support of the FMRIF core facility, specifically with the friendly help from Sean Marrett and Kenny Chung.
Patients recruitment: we thank Elaine Considine and Vivian Koo for their assistance with patient recruitment.
Funding: Laurentius Huber was funded from the NWO VENI project 016.Veni.198.032. The project was supported by the NINDS Intramural Research Program.
Ethics: We thank Avanti Iyer for help with the IRB protocol
References
1. Hallett M. Neurophysiology of dystonia: The role of inhibition. Neurobiol Dis. 2011;42(2):177-184. doi:10.1016/j.nbd.2010.08.025
2. Butterworth S, Sue F, Edward K, Francis M, Richard B, Guy VS. Abnormal cortical sensory activation in dystonia: An fMRI study. Mov Disord. 2003;18(6):673-682. http://dx.doi.org/10.1002/mds.10416.
3. Nelson AJ, Blake DT, Chen R. Digit-specific aberrations in the primary somatosensory cortex in writer’s cramp. Ann Neurol. 2009;66(2):146-154. doi:10.1002/ana.21626
4. Catalan MJ, Ishii K, Bara-Jimenez W, Hallett M. Reorganization of the Human Somatosensory Cortex in Hand Dystonia. J Mov Disord. 2012;5(1):5-8. doi:10.14802/jmd.12002
5. Bara-Jimenez W, Catalan MJ, Hallett M, Gerloff C. Abnormal somatosensory homunculus in dystonia of the hand. Ann Neurol. 1998;44(5):828-831. doi:10.1002/ana.410440520
6. Elbert T, Candia CAV, Altenmüller E, et al. Alteration of digital representations in somatosensory cortex in focal hand dystonia. Neuroreport. 1998;9(16):3571-3575.
7. Lu H, Golay X, Pekar JJ, van Zijl PCM. Functional magnetic resonance imaging based on changes in vascular space occupancy. Magn Reson Med. 2003;50:263-274. doi:10.1002/mrm.10519
8. Huber L, Finn ES, Handwerker DA, et al. Sub-millimeter fMRI reveals multiple topographical digit representations that form action maps in human motor cortex. Neuroimage. 2020;208:116463. doi:10.1101/457002
9. Pakenham D, Asghar M, Glover P, et al. Assessing somatotopic and mototopic organisation in Focal Hand Dystonia using high-resolution 7T fMRI. In: Proc. Joint Annual Meeting ISMRM. ; 2019:0361
10. Poser BA, Koopmans PJ, Witzel T, Wald LL, Barth M. Three dimensional echo-planar imaging at 7 tesla. Neuroimage. 2010;51(1):261-266. doi:10.1016/j.neuroimage.2010.01.108
11. Huber L, Poser BA, Bandettini PA, et al. LayNii: A software suite for layer-fMRI, NeuroImage. 2021;237 (August) doi:10.1016/j.neuroimage.2021.118091.
12. Stephan KE, Petzschner FH, Kasper L, et al. Laminar fMRI and computational theories of brain function. Neuroimage. 2019;(October):1-8. doi:10.1016/j.neuroimage.2017.11.001
13. McColgan P, Joubert J, Tabrizi SJ. The human motor cortex microcircuit: insights for neurodegenerative disease. Nat Rev Neurosci. 2020;3(4):1-15. doi:10.1038/s41583-020-0315-1
14. Schreiber S, Northall A, Weber M, et al. Topographical layer imaging as a tool to track neurodegenerative disease spread in M1. Nat Rev Neurosci. 2021;22(1):69. doi:10.1038/s41583-020-00405-9
15. Haarsma J, Kok P, Browning M. The promise of layer-specific neuroimaging for testing predictive coding theories of psychosis. Schizophr Res. 2020. doi:10.31234/osf.io/xeyv7
16. Wan Y, Qian C, Wen W, Zhang P. Layer-dependent amblyopic deficits in feedforward and lateral processing in human early visual cortex. In: OHBM; 2021:2632.
Figures
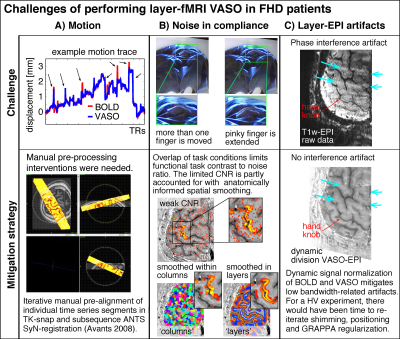
Figure 1: Selected examples of challenging aspects in the acquisition and processing of sub-millimeter VASO fMRI in FHD patients.
Head motion was mitigated with time consuming manual corrections. Limited finger specific functional CNR was mitigated with anatomically informed signal pooling within layers and columns respectively. Time constant EPI phase interference artifacts were mitigated by means of dynamic division of odd and even time points in SS-SI VASO. Intermittent ghosting could not be accounted for.
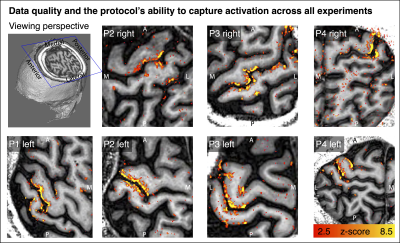
Figure 2: Tapping induced activation maps across patients.
Within the 30min functional experiments, enough data are obtained to extract significant VASO signal changes across all patients and hemispheres. The figures represent the signal without spatial smoothing in spatially upsampled in-plane resolution of 0.4mm (nominal resolution 0.75mm).
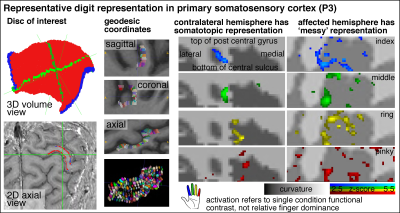
Figure 3: Cortical flattening of thin MRI slab
The first two columns exemplify how we flattened the cortical surface despite the fact that the thin slab does not fulfil common topology requirements of mesh-based analyses. Here, we imposed a local coordinate system in the distorted EPI data with LN2_MULTILATERATE.a Representative finger responses show somatotopic alignment in the contralateral hemisphere and less so in the affected hemisphere (smoothed across layers only).
a Gulban et al., OHBM, 2021; #1286
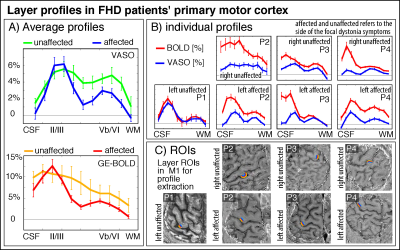
Figure 4: layer fMRI profiles in the primary motor cortex for BOLD and VASO in contralateral and affected hemisphere.
All profiles consistently show a larger superficial bias in GE-BOLD compared to VASO, while still showing clear indication of a secondary “bump” in the deeper output layers. Superficial layers in the affected hemispheres seem to be stronger activated compared to their contralateral counterparts. Signal pooling of layers was done from unsmoothed data.