2188
Ultra-high resolution human functional imaging using 10.5 Tesla1CMRR, University of Minnesota, Minneapolis, MN, United States, 2Department of Neurosurgery, University of Minnesota, Minneapolis, MN, United States
Synopsis
With the development of accelerated acquisition protocols, it is possible to achieve functional brain mapping with submillimeter resolution. This permits studying the human brain at the mesoscopic scale. Typical submillimeter images measure 0.8 mm. isotropic voxels, barely enough to study human functional mesoscopic responses. Here we acquire functional images at 10.5 T with the unprecedented spatial resolution of 0.4 mm. isotropic voxels. Using NORDIC to suppress thermal noise, we demonstrate the feasibility of achieving meaningful brain mapping at these ultra-high resolutions, where single voxels contains but a few thousand cells, further bridging the gap between fMRI and optical imaging
Introduction
Since its discovery, the Blood Oxygen Level Dependent (BOLD) based fMRI (1) has emerged as a primary tool for non-invasive studies of the human brain. With the development of efficient accelerated acquisition protocols, it has been possible to acquire BOLD fMRI with unprecedented spatial resolutions, reaching the mesoscopic scale, which had previously been accessible only in animal models using invasive techniques. These high-resolution studies target some of the most fundamental units of neural computations, such as layers and columns (e.g. 2; 3). These images typically utilize ~0.5µL voxel (e.g. 0.8 mm3 ), limited by SNR, and are only marginally adequate to study human mesoscopic scale organizations. This limitation is reflected in the Brain Initiative Working group goals, which challenged the MR community to achieve functional resolutions of ≤0.1µL voxels initially (4) and 0.01µL subsequently (5).Recently, using a denoising technique (NORDIC) that selectively suppresses thermal noise, the dominant noise-source at these resolutions, we approached the 0.1µL goal, demonstrating human functional brain mapping at 7T with 0.125 µL voxel volumes (6) Here, we exploit recent technological developments at 10.5T (7) together with NORDIC denoising to demonstrate 0.4x0.4x0.6 mm3 (0.096µL volume) and 0.4 mm isotropic (0.064µL volume) human GE BOLD fMRI images, surpassing the initial BRAIN Initiative goal of 0.1µL voxels and on the way to meeting the second more demanding target.Methods
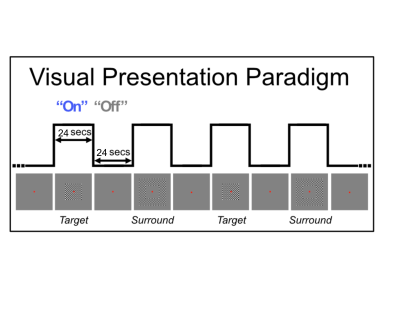
Functional MRI data were collected at 10.5T (Siemens) with a 16 transmit, 32 receive channel head coil. T2*-weighted images were collected with 2D GE EPI for the 0.4x0.4x0.6mm3 data (34 slices, TR:2576, iPAT:4, 5/8 Partial Fourier, TE:25 ms, Bandwidth:880Hz) and with 3D GE EPI for the 0.4mm isotropic data (40 slices, TR:75s, Volume acquisition time (VAT):3300ms, iPAT:3, 5/8 Partial Fourier, TE:21 ms, Bandwidth: 660Hz). This exact acquisition protocol was replicated at 7T. Shimming to improve B0 homogeneity over occipital regions was conducted manually. Functional preprocessing included slice time correction for the 2D acquisition, rigid body motion correction and low drift removals. We implemented a standard 24 seconds on/off visual block design paradigm with a center (i.e. target) and a surround checkerboard counter-phase flickering (at 6 Hz - Figure 1). Each run lasted approximately 5 minutes (3 trials x 2 conditions).Results
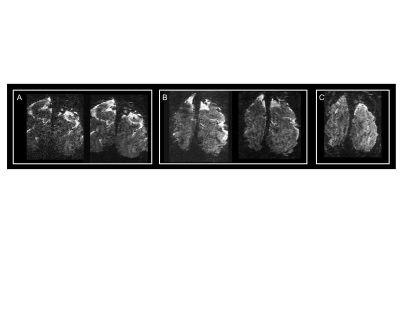
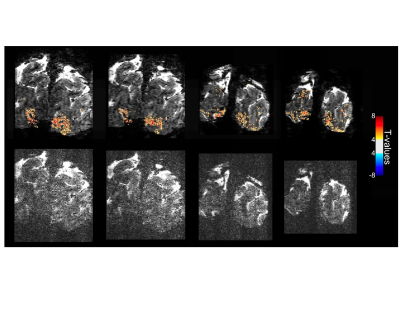
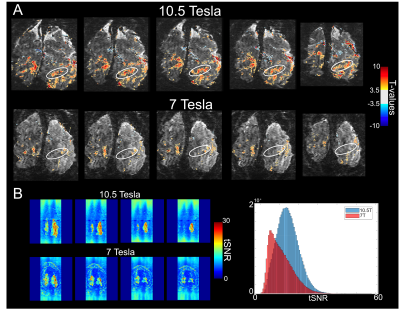
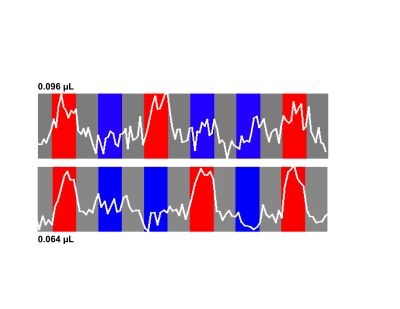
Figure 2 illustrates single slice GE EPI images from a single time point in the fMRI time series, from the 10.5T 0.096µL acquisition before (left) and after (right) NORDIC denoising (Fig. 2A); from the 10.5T 0.064µL acquisition before (left) and after (right) NORDIC denoising (Fig. 2B); and from the 7T 0.064µL acquisition after NORDIC denoising (Fig. 2C). Note the dramatic improvement in image SNR following NORDIC denoising and the superior image quality achievable at 10.5T for the 0.064µL resolution.Figure 3 shows t-maps (target & surround > 0) of a ~5 minute run for NORDIC (top) and Standard (bottom) at 10.5T, computed fort the for the 0.096µL acquisition superimposed on single EPI slices. At this t-threshold (t>3.5), no activation can be observed before NORDIC denoising. Figure 4A shows NORDIC-denoised t-maps (target & surround > 0) for 5 concatenated runs (~25 mins of data) for 10.5T (top) and 7T (bottom) for 0.064µL acquisitions. Consistent with the ~37% increase in tSNR at 10.5T (Figure 4B), significant portions of the expected retinotopic activation in the visual cortex (e.g. see circled regions) are only visible in the 10.5T images.Figure 5 shows examples of single-run, single-voxels time-courses for the two 10.5T acquisition protocols, displaying the expected temporal characteristics of BOLD responses.Discussion
In this work we demonstrate human functional brain mapping with unprecedented resolutions. We acquired GE BOLD fMRI images at 10.5T with voxel volumes spanning the <0.1 µL range. We successfully deal with the barrier represented by thermal noise, which is dominant at these resolutions, using NORDIC denoising on raw data which was demonstrated not to induce spatial blurring (6) We used a visual paradigm and exploited the retinotopic property of V1, which provided a ground truth for functional activation, allowing evaluation of the quality of the evoked BOLD reposes in space and time. Simultaneous use of NORDIC denoising and 10.5T were required for these functional maps, confirming the feasibility of performing human fMRI at such extremely high resolutions.Conclusion
We demonstrate the feasibility of functional mapping at 10.5 T with unprecedented spatial resolutions. We achieve the Brain Initiative goal by recording <0.1µL voxel volume fMRI in humans and move towards the 0.01µL goal set by the Brain Initiative 2.0 by recording the first ever human functional images with ~0.06µL voxel volume with ~5 to ~25 minutes acquisitions. At these ultra-high resolutions, a single voxel only contains a few thousand cells, further bridging the gap between fMRI and invasive optical imaging and heralding a major new expansion in human fMRI applications for neuroscience research. Further gains are expected with more advanced 10.5T coils with higher channel count receive arrays under development; preliminary data on these coils indicate significant SNR gains throughout the brain (see other 10.5T related abstracts).Acknowledgements
This work was supported by the NIH grants: U01 EB025144, P41 EB027061, RF1 MH116978.References
1. Ogawa, S., Tank, D. W., Menon, R., Ellermann, J. M., Kim, S. G., Merkle, H., & Ugurbil, K. (1992). Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proceedings of the National Academy of Sciences of the United States of America, 89(13), 5951–5955. https://doi.org/10.1073/pnas.89.13.59512.
2. Yacoub, E., Harel, N., & Ugurbil, K. (2008). High-field fMRI unveils orientation columns in humans. Proceedings of the National Academy of Sciences of the United States of America, 105(30), 10607–10612. https://doi.org/10.1073/pnas.08041101053.
3. Muckli, L., De Martino, F., Vizioli, L., Petro, L. S., Smith, F. W., Ugurbil, K., Goebel, R., & Yacoub, E. (2015). Contextual Feedback to Superficial Layers of V1. Current biology : CB, 25(20), 2690–2695. https://doi.org/10.1016/j.cub.2015.08.0574.
4. BRAIN 2025: A Scientific Vision (2014) [Available from: https://braininitiative.nih.gov/sites/default/files/pdfs/brain2025_508c.pdf.5.
5. From Cells to Circuits, Toward Cures (BRAIN 2.0) 2019 [Available from: https://braininitiative.nih.gov/sites/default/files/images/brain_2.0_6-6-19-final_revised10302019_508c.pdf.
6. Vizioli L, Moeller S, Dowdle L, Akçakaya M, De Martino F, Yacoub E, Uğurbil K. Lowering the thermal noise barrier in functional brain mapping with magnetic resonance imaging. Nature communications. 2021 Aug 30;12(1):1-5.
7. Tavaf, N., Lagore, R. L., Jungst, S., Gunamony, S., Radder, J., Grant, A., Moeller, S., Auerbach, E., Ugurbil, K., Adriany, G. & Van de Moortele, P. F., 2021. A self-decoupled 32-channel receive array for human-brain MRI at 10.5 T. Magn Reson Med 86, 1759-1772. Epub Date: 2021/03/30 PMID: 33780032 PMCID: PMC8184625
Figures