2184
Investigation of MR visibility control of water-based (CaTiO3) dielectric padding with iron oxide contrast agent in 7T human brain MRI1Department of Biomedical Engineering, Sungkyunkwan university, Suwon, Korea, Republic of, 2Intelligent Precision Healthcare Convergence, Sungkyunkwan University, Suwon, Korea, Republic of, 3Department of Data Science, Inha University, Incheon, Korea, Republic of, 4Center for Neuroscience Imaging Research,Institute for Basic Science, Suwon, Korea, Republic of
Synopsis
Ultra-high-field MRI provides improved signal-to-noise ratio (SNR) and tissue contrast. However, at the same time, it exhibits stronger RF field inhomogeneity compared to lower-field MRI. Although such inhomogeneity can be mitigated by customized RF coils and parallel transmit methodology, it is often difficult to translate these to clinical use. Recently, a simple and low-cost high dielectric-constant padding has been proposed to improve the quality of 7T brain images in many sequences. Here, we propose a novel approach to suppress the MR visibility of a water-based dielectric padding by controlled addition of iron oxide contrast agent in 7T human brain MRI.
Introduction
Clinical 7T MRI enables better diagnostics in the clinic by providing high SNR and enhanced tissue contrast. One of the challenges in human 7T MRI is the inhomogeneity of the radio frequency (RF) field, which occurs due to its short wavelength at the Larmor frequency (298 MHz). When we use a conventional circularly polarized RF transmit coil, the field inhomogeneity causes significant image shading in the cerebellar regions of the brain. Customized RF coils and parallel transmit methodology are effective to mitigate this problem. However, these approaches are not easily available for clinical use. Thus, a simple and low-cost alternative solution of high dielectric-constant padding has been used to improve 7T brain scans. 1 It is placed under the subject's head, and effectively increases the spin flip angle and signal sensitivity in the cerebellum. A padding made of an aqueous solution of calcium titanium oxide (CaTiO3, to be called CTO) is particularly easy to produce owing to its accessibility and low cost. Prior works showed that this approach enhanced 7T brain images in many sequences.1,2,3 In this study, we extend this method by suggesting an effective way to suppress the MR visibility of a CTO dielectric padding. Specifically, we demonstrate dramatic reduction in visibility by addition of a commercial iron oxide nanoparticle T2* contrast agent (Molday ION, BioPal, Worchester, MA, USA) to the water-based padding.Methods
T2* estimation of CTO-based dielectric padding with 2.7:1 weight ratio of CTO and deionized water was conducted prior to the addition of MION. For the estimation, 2D-GRE images with different TEs were acquired (Fig.1A). We then manually selected the region-of-interest (ROI) by considering the signal homogeneity of images, and the values of ROIs were used to fit T2* (Fig.1B). The proper compound ratio of MION and CTO-based dielectric padding was calculated from Eq. 1 to make T2* of this mixture less than 1 ms.1/R2*(new padding) = 1/R2* (original) + 1/R2* (MION) [Eq.1]
The relaxivity r2* of the MION at 7T was assumed to be 178 [s-1mM-1], from a previous study4,5, and the effect of increased susceptibility due to the addition of MION was also considered. After making new CTO dielectric padding with an optimal ratio of MION, in-vivo human brain images with dielectric padding were acquired using 2D-GRE and MP2RAGE sequences. We considered the occipital regions as the ROI to quantitatively assess the signal intensity and B0 inhomogeneity.
Results
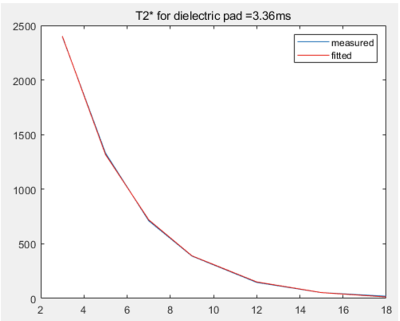
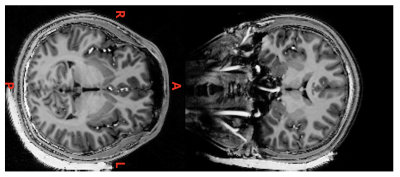
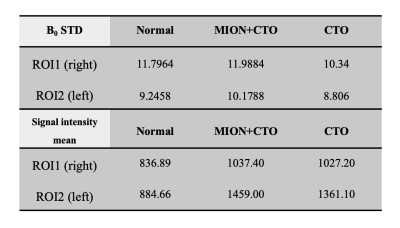
Figure 2 shows the fitted T2* value of CTO-based dielectric padding without MION addition, which was 3.36 ms. We could conclude that additional MION should have R2* greater than about 700 s-1. This led to the addition of 4 mM of MION to the original CTO-based dielectric padding making process. As a result, 2.29 ml of MION per 100 ml deionized water was added. Figure 3 shows MP2RAGE images of the human brain with original CTO padding and improved CTO padding with MION. The signal of improved CTO padding was significantly suppressed and rarely visible in MP2RAGE and 2D-GRE images. Figure 4 illustrates the B maps of 2D-GRE images, and B0 standard deviations of the ROIs are represented in Table 1. The mean signal intensity values showed that in Table 2 were calculated using 2D-GRE images without any post-processing steps. The mean signal intensity values show that low-visibility CTO-based dielectric padding with MION keeps acting as a signal amplifier despite additional MION. Although B0 standard deviation was slightly increased, the increase of magnetic susceptibility was still acceptable in terms of image quality.Conclusion
We confirmed that the addition of MION to CTO-based dielectric padding can effectively reduce the visibility of padding in MP2RAGE with TE as short as 2 ms, while a minor increase in susceptibility-induced B0 inhomogeneity was observed with minimal effect in GRE image quality. We conclude that the visibility of water-based CTO padding can be controlled by adding a balanced amount of iron oxide contrast agent with respect to magnetic susceptibility changes.Acknowledgements
This work was supported in part by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) under Grant 2019R1A2C1006448 and in part by the Institute for Basic Science under Grant IBS-R015-D1.References
1. Teeuwisse, W. M., Brink, W. M., & Webb, A. G. (2011). Quantitative assessment of the effects of high-permittivity pads in 7 Tesla mri of the brain. Magnetic Resonance in Medicine, 67(5), 1285–1293.
2. Vaidya, M. V., Lazar, M., Deniz, C. M., Haemer, G. G., Chen, G., Bruno, M., Sodickson, D. K., Lattanzi, R., & Collins, C. M. (2018). Improved detection of fmri activation in the cerebellum at 7t with dielectric pads extending the imaging region of a commercial head coil. Journal of Magnetic Resonance Imaging, 48(2), 431–440.
3. Brink, W. M., van der Jagt, A. M. A., Versluis, M. J., Verbist, B. M., & Webb, A. G. (2014). High permittivity dielectric pads improve high spatial resolution magnetic resonance imaging of the inner ear at 7 T. Investigative Radiology, 49(5), 271–277.
4. Brewer KD, Spitler R, Lee KR, Chan AC, Barrozo JC, Wakeel A, et al. Characterization of Magneto-Endosymbionts as MRI Cell Labeling and Tracking Agents. Mol Imaging Biol. 2018; 20(1):65–73.
5. Kim J-H, Kim J-H, Lee S-H, Park J, Lee S-K (2019) Fabrication of a spherical inclusion phantom for validation of magnetic resonance-based magnetic susceptibility imaging. PLoS ONE 14(8): e0220639
Figures