2176
Dual-echo single-shot SMS EPI for improved MR thermometry1School of Biomedical Engineering and Imaging Sciences, Faculty of Life Sciences and Medicine, King's College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom, 3Siemens Healthcare GmbH, Erlangen, Germany, 4Siemens Medical Solutions USA Inc., Malvern, PA, United States
Synopsis
MR thermometry using dual-echo single-shot EPI was evaluated in a phantom. Dual-echo EPI resulted in better temperature stabilities than the single echo acquisitions. This sequence was successfully combined with SMS, which also resulted in higher temperature stability than the single echo acquisitions.
Introduction
MR thermometry plays an important role for the real-time monitoring of MRI-guided ablation therapies. Fast MR thermometry with short TR (<100 ms)1 allows the acquisition of motion-free images in moving organs using single-shot EPI. Precision of MR-thermometry is directly related to TE which should ideally be matched to the T2* of the targeted tissue. Multi-echo sequences can improve the precision of thermometry as previously demonstrated using GRE or segmented EPI acquisitions2. In this study, we sought to investigate the potential of dual-echo (dTE) single-shot EPI to improve the precision of MR-thermometry and combine it with simultaneous multi-slice (SMS) imaging3 to provide increased spatial coverage.Methods
A diagram of the proposed prototype SMS dual-echo single-shot EPI sequence is shown in Figure 1. SMS EPI was achieved using a blipped CAIPI approach4. The imaging parameters were optimized so the second TE matches the targeted T2* time. Temperature maps were obtained from the phase images using the PRFS method5. Temperature maps were reconstructed for each TE and were combined using weights as described by Odéen and Parker2:$$$w_j (\bar{r})=a (\textrm{TE}_j |m_j (\bar{r})|)^2$$$,
where $$$\textrm{TE}_j$$$ was the echo time of the j-th image, $$$m_j (\bar{r})$$$ the complex MRI image value at position $$$\bar{r}$$$, and $$$a$$$ the normalization factor such that $$$∑_j w_j (\bar{r})=1$$$.
The sequence was evaluated in a standardized phantom (T1mes)6 using a 1.5T scanner (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany). Of this phantom, 4 vials were considered that had T2* values between 41 and 47 ms (vials D, F, G, and I, with T1 values between 300 and 1000 ms).
In the first experiment, the prototype dTE EPI sequence was acquired in single band mode in a single slice (TE1 = 20 ms, TE2 = 57 ms, TR = 117 ms, FOV 180x180 mm2, voxel size 1.6x1.6 mm2, slice thickness 6 mm, BW 1540 Hz/pixel, flip angle 35˚, partial Fourier 75%, GRAPPA factor 2, number of dynamics: 150) and compared to a conventional single-echo EPI acquisition with TE = 20 ms, TR = 80 ms and otherwise matching imaging parameters. Scans in the second experiment were acquired with the same parameters, but now acquiring 2 slices using SMS factor 2 for both the prototype dTE and single TE acquisitions (slice distance 6 mm).
Stability maps were calculated as the standard deviation over time of the temperature in each voxel. Stability maps were produced using temperature maps derived from single echo only or using the proposed combined dTE.
Results
In the single slice experiment, combining the dual-echo data always led to the best temperature stability (Figure 2), with an average increase in stability of 19% compared to the temperature stability obtained from using only the 2nd echo in the dTE sequence. The faster conventional sequence always showed the lowest stability.In the experiment with SMS, the combined dual-echo data also outperformed the other acquisitions in all vials (Figure 3). Although the overall stability was lower than in the single band experiment, the improvement due to adding TE2 to TE1 was similar at 21% and 20% in these slices.
Discussion
Temperature stability using dTE was consistently highest in both experiments. TE2 was longer than the T2* of the vials, it remains to be seen if larger improvements are possible with closer matching TE2. These experiments were conducted in a static phantom, the benefit of the dTE single-shot EPI technique in-vivo remains to be demonstrated.Conclusion
MR thermometry using dual-echo single-shot EPI resulted in better temperature stabilities than the single echo acquisitions. This sequence was successfully combined with SMS which also resulted in higher temperature stabilities than the single echo acquisitions.Acknowledgements
This work was supported by the Engineering and Physical Sciences Research Council (EPSRC) grant (EP/R010935/1), the British Heart foundation (BHF) grant (PG/19/11/34243), The Innovate UK grant (68539), the Wellcome EPSRC Centre for Medical Engineering at Kings College London (WT 203148/Z/16/Z), the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.References
1. Roujol S, Ries M, Quesson B, Moonen C, Denis de Senneville B. Real-time MR-thermometry and dosimetry for interventional guidance on abdominal organs. Magn Reson Med. 2010;63(4):1080-1087. doi:10.1002/mrm.22309
2. Odéen H, Parker DL. Improved MR thermometry for laser interstitial thermotherapy. Lasers Surg Med. 2019;51(3):286-300. doi:10.1002/lsm.23049
3. Borman PTS, Bos C, de Boorder T, Raaymakers BW, Moonen CTW, Crijns SPM. Towards real-time thermometry using simultaneous multislice MRI. Phys Med Biol. 2016;61(17):N461-N477. doi:10.1088/0031-9155/61/17/N461
4. Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med. 2012;67(5):1210-1224. doi:10.1002/MRM.23097
5. Rieke V, Pauly KB. MR thermometry. J Magn Reson Imaging. 2008;27(2):376-390. doi:10.1002/jmri.21265
6. Captur G, Gatehouse P, Keenan KE, et al. A medical device-grade T1 and ECV phantom for global T1 mapping quality assurance - the T1 Mapping and ECV Standardization in cardiovascular magnetic resonance (T1MES) program. J Cardiovasc Magn Reson. 2016;18(1):1-20. doi:10.1186/s12968-016-0280-z
Figures
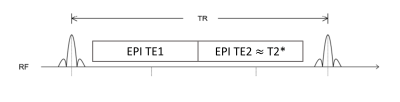
Figure 1. Schematic diagram of dual-echo single-shot EPI MR thermometry. Within one repetition time, two images are acquired. One image with a short echo time and one image with an echo time matching the T2* of the tissue under investigation.
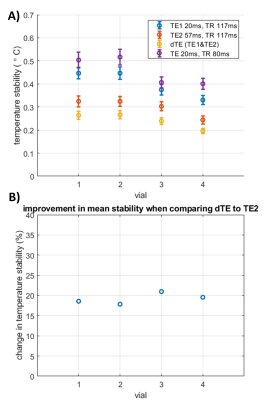
Figure 2. Temperature stability study using single band acquisitions (Experiment 1). A) Temperature stability (mean±sd) measured in a single slice in the different vials of the T1mes phantom. B) Improvement in mean stability due to combining the temperature maps of TE1 to TE2 using the dTE method, compared to the stability of temperature maps acquired with TE2 only.
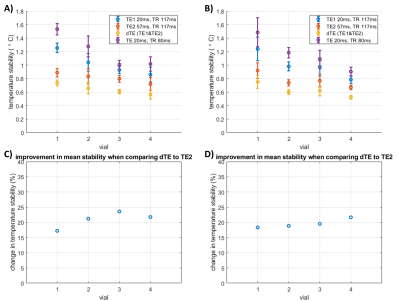
Figure 3. Temperature stability study using SMS acquisitions (Experiment 2). A&B) Temperature stability (mean±sd) measured in two slices acquired with SMS acceleration in the different vials of the T1mes phantom. C&D) Improvement in mean stability due to combining the temperature maps of TE1 to TE2 using the dTE method, compared to the stability of temperature maps acquired with TE2 only.