2174
Towards MR-guided Hyperthermia at Low Field: a Proof-of-Concept Investigation1Department of Biomedical Engineering, University of Basel, Allschwil, Switzerland
Synopsis
MR-guided hyperthermia at low magnetic field is challenging due to the inherently low SNR, which typically calls for multiple signal averages. Dedicated coils are crucial at this field regime, to maximise signal acquisition. In this study we built a custom RF coil which integrates a water-based heating system to reproduce hyperthermia conditions in hands. Using a fast T1-mapping sequence, we could successfully monitor the temperature variations generated in a phantom over 120min. A first in vivo employment in a volunteer’s hand at room temperature was also shown, achieving a spatial and temporal resolution compatible with hyperthermia requirements.
Introduction
Hyperthermia treatments rely on exposing the tissue to mild temperatures (39-43°C range) for 30 to 90min1,2 in parallel to anti-tumour treatments, such as chemotherapy and radiotherapy, in order to enhance their efficacy3–7. Accurate mapping of the local temperature distribution in the treated region is crucial to assess the deposited thermal dose and to avoid hot spots. Although MR thermometry established itself as a fast and reliable modality for temperature mapping, to this day invasive temperature probes are still favoured8,9. This is mainly due to the high costs associated to the use of clinical MR scanners, the limited availability of fully integrated MRI/hyperthermia systems, and the technique limitations in adipose tissues or moving organs10. Low field scanners could represent a valid alternative for MR thermometry, thanks to the reduced economic footprint and increased flexibility. In this work we present a proof-of-concept set-up for low field hyperthermia in hands. A custom-made RF coil was developed, with an integrated water-circulating for heat delivery. Using a T1-based temperature mapping sequence, we could successfully follow the temperature variations in a phantom heated up to 50°C over 120min. Using the same sequence/set-up, a T1 map of a volunteer’s hand could be acquired at room temperature in 4min58s.Methods
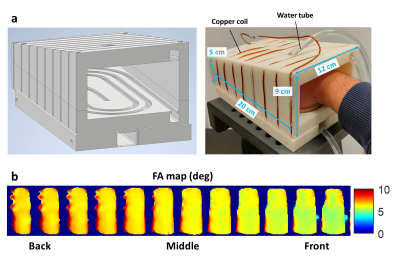
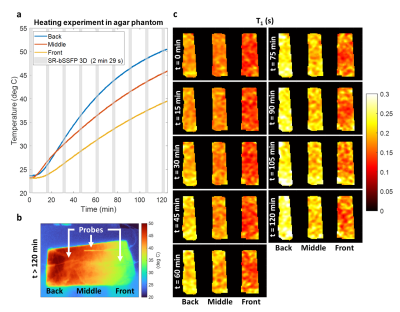
To reproduce hyperthermia conditions, a heating-box based on circulating hot water was built (Fig.1). A custom transceiver cylindrical RF coil with inductive coupling was wound around a 3D-printed case shaped to accommodate a hand while maximising the filling factor. The electrical characteristics measured with a VNA (E5061B-3L4, Keysight Technologies, USA) were as follows: working frequency=4.35MHz, S11=-7dB, BW=18kHz, Q=237. A flip angle map was measured using the LAM method11, with a nominal flip angle (FA) of 5°. A PVC-tube (outer/inner diameters=8/6mm), connected to a temperature-controlled water circulating system (PD7LR-20, PolyScience AG, Switzerland), was integrated in the case to generate homogeneous heating. A proof-of-concept hyperthermia experiment was carried out using an agar sample (3.5%w/w agar, 0.3mM MnCl2, 0.5%w/w ADBAC) fully fitting in the case. The temperature-control system was set to 65°C for 120min. Ground truth measures were provided by three temperature probes (OTP-A temperature sensors, Althen, Germany) inserted in the phantom (Fig.2b). T1 maps were acquired every 15 min using a Look-Locker-based mapping technique with a bSSFP readout and an initial saturation recovery excitation. Each acquisition lasted 2min29s with the following imaging parameters: FA=5°, matrix=64$$$\times$$$27$$$\times$$$11, resolution=3$$$\times$$$3$$$\times$$$15mm3, undersampling factor=50%, TR/TE=1000/4.1ms, TI=8ms, pixel bandwidth=312Hz. The same sequence parameters were also employed to acquire one in vivo T1 map of a volunteer’s in 4min58s (TR=2000ms). All images were acquired using a resistive biplanar MRI system (Bouhnik S.A.S., France) operating at 0.1T.Results
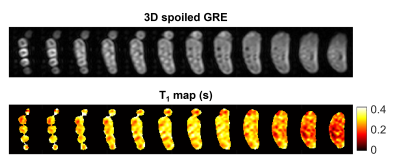
The three temperature traces obtained from the agar phantom show a monotonic temperature rise (Fig.2a). T1 maps at the different time points are displayed in Fig.2c. Temperature values computed from T1 maps are in good agreement with the ground truth measures (not shown). Fig.3 presents the T1 map acquired in vivo at room temperature, together with an anatomical image acquired using a 3D spoiled GRE sequence with the same resolution for comparison.Discussion
The water-based hyperthermia set-up could successfully achieve temperatures as high as 50°C in the agar phantom over the 120-min exposure. While the probes reported comparable temperatures at the first time point, the divergence of the three traces over time suggests a temperature gradient within the phantom. This was confirmed at the end of the experiment by a thermal camera image (Fig.2b). Although this could have been predicted due to the non-uniform geometry of the box and phantom, further iterations in the design are required to achieve a more even heat distribution. The acquired T1 maps proved able to capture the temperature variations in the front, middle and back of the phantom. An increasing gradient was also observed between subsequent time points. A first in vivo map of a volunteer’s hand was obtained in 4min58s. The acquisition time was doubled in vivo because of a doubled TR to better sample longer T1s expected in the human body. Despite the coarse resolution, the main anatomical structures of the hand could be identified, with T1s of different tissue types ranging roughly between 200ms to 400ms. Generally shorter T1s were found towards the wrist, however we believe that this could be ascribed to inhomogeneities in the B1+ profile (Fig1.b). Indeed, a similar gradient could also be identified in the phantom in the absence of external heating (Fig2.c). Although T1 modelling appears fully independent on the employed flip angle α (provided that α$$$\rightarrow$$$0)12 further investigation into the impact of the flip angle on T1 recovery using the proposed sequence is mandatory.Conclusions
In this proof-of-concept investigation we could successfully monitor a mimicked hyperthermia experiment in phantom using a 0.1T scanner. With the presented set-up, specifically designed for hands, we could acquire maps both in phantom and in vivo with a spatial and temporal resolution compatible with the general requirements for hyperthermia2. T1 dependence on temperature needs to be investigated in vivo to provide temperature maps and this step will follow once the required ethical approval is obtained.Acknowledgements
This research was funded by the Swiss National Science Foundation Grants No. 170575, No. 186861 and No. 198905. The authors would like to thank the COST Action CA15209 for the insightful discussions.References
1Overgaard J, Suit HD. Time-Temperature Relationship in Hyperthermic Treatment of Malignant and Normal Tissue in Vivo. Cancer Res. 1979;39(8):3248-3253.
2Feddersen T V, Hernandez-Tamames JA, Franckena M, van Rhoon GC, Paulides MM. Clinical Performance and Future Potential of Magnetic Resonance Thermometry in Hyperthermia. Cancers (Basel). 2020;13(1):31. doi:10.3390/cancers13010031
3Vujaskovic Z, Song CW. Physiological mechanisms underlying heat-induced radiosensitization. Int J Hyperth. 2004;20(2):163-174. doi:10.1080/02656730310001619514
4Datta NR, Gómez Ordóñez S, Gaipl US, et al. Local hyperthermia combined with radiotherapy and-/or chemotherapy: Recent advances and promises for the future. Cancer Treat Rev. 2015;41(9):742-753. doi:10.1016/j.ctrv.2015.05.009
5Colombo R, Salonia A, Leib Z, Pavone-Macaluso M, Engelstein D. Long-term outcomes of a randomized controlled trial comparing thermochemotherapy with mitomycin-C alone as adjuvant treatment for non-muscle-invasive bladder cancer (NMIBC). BJU Int. 2011;107(6):912-918. doi:10.1111/j.1464-410X.2010.09654.x
6Hurwitz M, Stauffer P. Hyperthermia, radiation and chemotherapy: The role of heat in multidisciplinary cancer care. Semin Oncol. 2014;41(6):714-729. doi:10.1053/j.seminoncol.2014.09.014
7Horsman MR, Overgaard J. Hyperthermia: a Potent Enhancer of Radiotherapy. Clin Oncol. 2007;19(6):418-426. doi:10.1016/j.clon.2007.03.015
8Fani F, Schena E, Saccomandi P, Silvestri S. CT-based thermometry: An overview. Int J Hyperth. 2014;30(4):219-227. doi:10.3109/02656736.2014.922221
9Kok HP, Wust P, Stauffer PR, Bardati F, van Rhoon GC, Crezee J. Current state of the art of regional hyperthermia treatment planning: A review. Radiat Oncol. 2015;10(1):1-14. doi:10.1186/s13014-015-0503-8
10Odéen H, Parker DL. Magnetic resonance thermometry and its biological applications – Physical principles and practical considerations. Prog Nucl Magn Reson Spectrosc. 2019;110:34-61. doi:10.1016/j.pnmrs.2019.01.003
11Balezeau F, Eliat PA, Cayamo AB, Saint-Jalmes H. Mapping of low flip angles in magnetic resonance. Phys Med Biol. 2011;56(20):6635-6647. doi:10.1088/0031-9155/56/20/008
12Schmitt P, Griswold MA, Jakob PM, et al. Inversion Recovery TrueFISP: Quantification of T1, T2, and Spin Density. Magn Reson Med. 2004;51(4):661-667. doi:10.1002/mrm.20058
Figures