2168
Validation of a 3D MR temperature imaging sequence for use during breast magnetic resonance guided focused ultrasound treatments1Biomedical Engineering, University of Utah, Salt Lake City, UT, United States, 2Radiology and Imaging Sciences, University of Utah, Salt Lake City, UT, United States
Synopsis
This work presents a 3D MR temperature imaging (MRTI) sequence for breast MRgFUS. A 3D gradient echo with segmented echo planar imaging readout sequence with time-varying drift correction was used in female volunteers and tissue-mimicking phantoms. Temperature changes due to FUS heating in phantoms were measured with fiberoptic probes and compared to MRTI. Non-heating volunteer data was also acquired. Temperature in the phantom studies was accurate compared to FO probes with an RMSE of 1.0°C. Mean precision and accuracy of 1.05 and 1.29°C were measured in healthy volunteers. This sequence provides accurate and precise 3D temperature measurements for breast MRgFUS.
Introduction
Treatment of localized breast cancer has evolved from radical mastectomy to breast-conserving therapies. Several minimally invasive options, including MR guided focused ultrasound (MRgFUS), have been used to treat breast cancer. MRgFUS can be monitored in real-time with proton resonance frequency (PRF) based MR temperature imaging (MRTI). While PRF MRTI has been found effective for several applications, monitoring MRgFUS breast cancer treatments presents unique challenges, including respiratory artifacts and the highly heterogeneous nature of breast tissue. PRF MRTI sequences applied to date in in clinical breast MRgFUS treatments have not addressed both the above issues1–3. Use of 3D MRTI may additionally allow improved treatment efficacy while minimizing off-target damage. This work presents initial validation of a 3D PRF MRTI sequence for use in MRgFUS breast cancer treatments.Methods
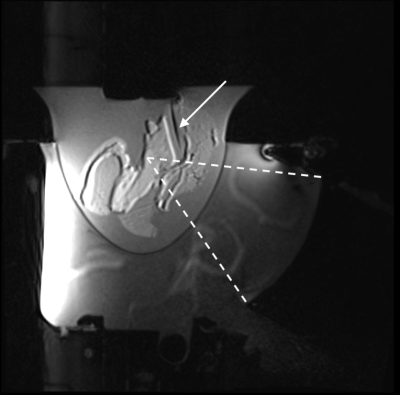
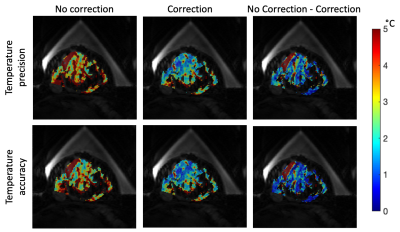
A 3D gradient echo with segmented echo planar imaging readout MR temperature imaging sequence was implemented on a 3T scanner (Siemens PrismaFIT). Images were acquired (2x1x3 mm voxel spacing, TR/TE/FA: 41ms/12ms/13°, 10 slices, 150x160x30 mm FOV, ETL=7, fat saturation) with a breast MRgFUS system with an integrated 8 channel MR receive coil2. Time-varying magnetic field drift was measured directly from the phase difference between each k-space point and its baseline phase averaged over 5 baseline k-space measurements. Precision in phase difference is improved by taking the complex mean overreadout, slice, and coil k-space dimensions. These phase drift values were subtracted from the k-space measurements for all acquisitions. The images were reconstructed with optimal coil combine using an inverse covariance matrix derived from coil noise4. Images were zero-filled interpolated to 1mm isometric voxel spacing. Temperatures were calculated using the PRF method5.Experiments were conducted in tissue-mimicking phantoms and healthy female volunteers to evaluate the precision and accuracy of the presented temperature sequence. Tissue mimicking phantoms: Two phantoms with degassed salt pork embedded in non-attenuating gelatin were constructed in an acoustically transparent breast-shaped mold (Figure 1). Catheters were placed in muscle tissue, and fiberoptic (FO) temperature probes (Neoptix T1™) were inserted into the catheters with the probe tip extending 3-5 mm from the catheter end. Probe tip locations were identified with T1-weighted MR images. Each probe location was heated with MRgFUS (30 s sonication, 51-67 acoustic W). Temperature response was assessed using synchronized MRTI and FO probe measurements. To mitigate the influence of viscous heating artifact on FO temperature measurements6, MRTI and FO probe measurements were compared after the FUS had been off for >10 s. The accuracy of the MRTI temperature change was assessed by comparing the peak MRTI measurement near the catheter tip and the associated FO probe temperature change. Regression and Bland-Altman analyses were performed. Healthy female volunteers: Three healthy female volunteers (Figure 2) were scanned under non-heating conditions. Sequential (N=11-12) MRTI sequences were obtained. The precision of volunteer data was calculated as the mean of the standard deviation through time for all fibroglandular tissue voxels. Accuracy was calculated as the RMSE between all fibroglandular tissue voxels and 0°C, and the mean spatial accuracy was assessed over all voxels. Precision and accuracy of volunteer data were evaluated with and without time-varying drift correction.
Results
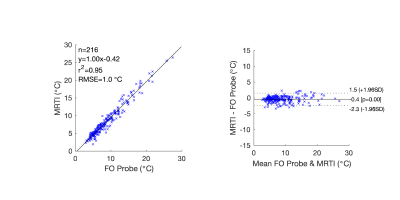
In heating studies with tissue-mimicking phantoms, 216 comparison points were recorded using MRTI and FO probes. The temperature measurements were strongly correlated (R2=0.95, RMSE=1.0°C) with a line with a slope of 1.00 over a temperature rise range of 2.6 to 26.9°C (Figure 3). The difference between measurements had limits of agreement -0.4±1.9°C (95% confidence interval). In three healthy human volunteers, the presented 3D MRTI sequence demonstrated a mean precision and accuracy of 1.05 and 1.29°C in fibroglandular tissue. The time-varying drift correction method showed a 46.1 and 43.4% improvement in precision and accuracy (Table 1, Figure 4), respectively, relative to the non-corrected condition.Discussion
MRTI measurements of temperature over time agreed closely with those made by FO probes. On average, temperature changes measured with MRTI were slightly less than those with FO probes and were accurate within 2.3°C over the range tested. While precision and accuracy varied among the limited number of volunteers, the time-varying drift correction substantially improved precision and accuracy for all. Volunteer 2 was least accurate and precise due to the large amount of fibroglandular tissue located close to the chest wall, where respiratory artifacts are more pronounced. While any MRTI sequence that utilizes the PRF technique cannot measure temperature in fat, all tissue targeted for treatment would be aqueous, allowing treatment efficacy to be improved.Conclusion
MRTI measurements agreed closely with FO probe measurements during heating studies over a clinically relevant range of temperatures and demonstrated accuracy and precision in healthy volunteers. This method provides accurate 3D MRTI measurements of aqueous tissue in a fatty environment and may allow for improved efficacy and precision of MRgFUS breast cancer treatments while preserving off-target tissue.Acknowledgements
NIH R37CA224141 and S10OD018482References
1. Furusawa H, Namba K, Thomsen S, et al. Magnetic Resonance-Guided Focused Ultrasound Surgery of Breast Cancer: Reliability and Effectiveness. J. Am. Coll. Surg. 2006;203:54–63 doi: 10.1016/j.jamcollsurg.2006.04.002.
2. Payne A, Merrill R, Minalga E, et al. A Breast-Specific MR Guided Focused Ultrasound Platform and Treatment Protocol: First-in-Human Technical Evaluation. IEEE Trans. Biomed. Eng. 2021;68:893–904 doi: 10.1109/TBME.2020.3016206.
3. Merckel LG, Knuttel FM, Deckers R, et al. First clinical experience with a dedicated MRI-guided high-intensity focused ultrasound system for breast cancer ablation. Eur. Radiol. 2016;26:4037–4046 doi: 10.1007/s00330-016-4222-9.
4. Roemer PB, Edelstein WA, Hayes CE, Souza SP, Mueller OM. The NMR phased array. Magn. Reson. Med. 1990;16:192–225 doi: https://doi.org/10.1002/mrm.1910160203.
5. De Poorter J, De Wagter C, De Deene Y, Thomsen C, Ståhlberg F, Achten E. Noninvasive MRI thermometry with the proton resonance frequency (PRF) method: in vivo results in human muscle. Magn. Reson. Med. 1995;33:74–81 doi: 10.1002/mrm.1910330111.
6. Morris H, Rivens I, Shaw A, Haar G Ter. Investigation of the viscous heating artefact arising from the use of thermocouples in a focused ultrasound field. Phys. Med. Biol. 2008;53:4759–4776 doi: 10.1088/0031-9155/53/17/020.
Figures