2161
High temporal-resolution MRI during mild-cold exposure enables the assessment of brown adipose tissue with a low inter-image variability
Aashley S.D. Sardjoe Mishre1,2, Maaike E. Straat2, Borja Martinez-Tellez2, Mariëtte R. Boon2, Oleh Dzyubachyk3,4, Andrew G. Webb1, Patrick C.N. Rensen2, and Hermien E. Kan1
1Department of Radiology, C.J. Gorter Center for High Field MRI, Leiden University Medical Center, Leiden, Netherlands, 2Department of Medicine, Division of Endocrinology and Einthoven Laboratory for Experimental Vascular Medicine, Leiden University Medical Center, Leiden, Netherlands, 3Department of Radiology, Division of Image Processing (LKEB), Leiden University Medical Center, Leiden, Netherlands, 4Department of Cell and Chemical Biology, Electron Microscopy section, Leiden University Medical Center, Leiden, Netherlands
1Department of Radiology, C.J. Gorter Center for High Field MRI, Leiden University Medical Center, Leiden, Netherlands, 2Department of Medicine, Division of Endocrinology and Einthoven Laboratory for Experimental Vascular Medicine, Leiden University Medical Center, Leiden, Netherlands, 3Department of Radiology, Division of Image Processing (LKEB), Leiden University Medical Center, Leiden, Netherlands, 4Department of Cell and Chemical Biology, Electron Microscopy section, Leiden University Medical Center, Leiden, Netherlands
Synopsis
Brown adipose tissue (BAT) is considered to be a potential therapeutic target against cardiometabolic diseases. Activated BAT combusts intracellular fatty acids leading to a reduction in fat fraction . Both cold exposure and pharmacological stimuli can activate BAT, but the short-term dynamics of BAT activation are unknown. To assess supraclavicular BAT fat fraction dynamics during cold-exposure, we developed a 1-minute-time-resolution MRI protocol using breath-holds and co-registration to minimize motion-artefacts. We demonstrated the validity and feasibility of our image analysis method, and found an inter-image variability of less than 0.1% fat fraction.
Introduction
Brown adipose tissue (BAT) is involved in energy metabolism by combusting glucose and fatty acids to produce heat. BAT activation is a potential treatment against cardiometabolic diseases1,2. Cold exposure is the most established physiological stimulus to activate BAT very rapidly3, but pharmacological alternatives have also been proposed4. BAT activity is usually indirectly quantified via glucose uptake using [18F]fluorodeoxglucose (FDG)-PET-CT, but this modality has a poor temporal resolution due to radiation exposure5. MRI of the supraclavicular BAT region (scBAT) is a promising radiation-free alternative6. Most scBAT MRI studies have either used pre- and post-cooling assessments of the MRI-derived fat fraction (FF) or studied FF dynamics at a low temporal resolution7–11. This does not provide sufficient insights into the short-term dynamics of BAT activation, which may be needed for assessing the kinetics of BAT stimuli. Here, we developed an MRI protocol with a high temporal resolution for assessing scBAT FF, minimized variability due to breathing by applying non-rigid image registration, and applied these in a cold exposure study.Methods
Ten healthy volunteers (age: 24.8±3.0 years; BMI: 21.2±2.1kg/m2) underwent a standardized cooling procedure for BAT activation using a water-circulating blanket. After 20 minutes at thermoneutrality (32°C), the temperature was set to 18°C for one hour. Images were acquired at 3T using a 16-channel anterior and 12-channel posterior array and a 16-channel head-and-neck coil. Scans were acquired during the last 10 minutes of thermoneutrality and for 60 minutes during cooling using a 3D gradient-echo 12-point Dixon sequence: TR/TE1/ ΔTE/FA/resolution/FOV/breath-hold duration=12ms/1.12ms/ 0.87ms/3°/2.1 mm isotropic/400x×229×134 mm3/16 s, mDIXONQuant and scanner reconstructions. The acquisition time per scan was 1.03 mins. For analysis, we co-registered first-echo magnitude images of each dynamic to the first thermoneutral scan (reference scan) using Elastix12 (Fig. 1). FF of the scBAT depot was obtained from ROIs, which were coarsely delineated on the reference scan and using a mutual FF thresholding approach, wherein voxels were included if their FF was above 30% in both the reference scan and in the registered dynamic (Fig. 1). Voxel-wise FF differences between the reference scan and each dynamic i: ΔFFi (x,y,z) = FFi (x,y,z) - FFTN1 (x,y,z) were calculated and averaged over the ROI. ROIs were also drawn in the trapezius muscle for reference. The validity of the co-registration was assessed for each subject by registering the reference scan to each dynamic, and applying the inverse transform to deform the registered reference scan back to its original coordinates, and then analyzing the voxel-wise FF overlap in the scBAT area. The feasibility of the registration was determined by assessing scBAT FF dynamics and ROI sizes between registered and non-registered data. Subsequently, we compared the scBAT FF dynamics to the FF dynamics obtained in the trapezius muscle. The validity, feasibility and reference tissue analyses were evaluated by quantifying the intra-individual variability along the FF dynamics. This was done for all subjects by calculating a moving average using a [-3,3] time window along the FF dynamics, after which the mean squared error (MSE) of the residuals was calculated between the moving average and the FF data. In the feasibility analysis, we also used the moving average method to calculate the MSE for averaged scBAT FF dynamics of registered and non-registered data as a measure for the inter-individual variability.Results
All subjects were able to adhere to the protocol. The validation analysis resulted in small FF differences over time (MSE=0.003%±0.002%; Fig. 2A). The feasibility analysis resulted in lower MSE values for registered scBAT FF dynamics compared to non-registered data (MSE: 0.09%±0.08% versus MSE:0.14%±0.12%; Fig. 2B-C). scBAT ROIs were on average larger for registered data compared to non-registered data (8259±3272 versus 7571±2378 voxels). The MSE values for the averaged scBAT FF dynamics were 0.008% and 0.02% for registered and non-registered data, respectively. scBAT FF showed a gradual decrease in response to mild-cooling, whereas no response was seen in the trapezius muscle (MSE=0.02%±0.02%; Fig. 3).Discussion
Our data show a high registration accuracy, as evidenced by the low variability in the validation analysis. Also, the registration feasibility showed a lower variability along the FF dynamics in registered versus non-registered data. This difference is likely the result of motion-induced variation, which increases spatial mismatches between the dynamic image and the reference image, and decreases the number of co-located voxels with a FF higher than 30%. On average, registered scBAT FF dynamics showed a 2.5 lower inter-individual variability compared to non-registered FF dynamics. The absence of any dynamic FF pattern in skeletal muscle indicates that the scBAT FF response is not influenced by temporal changes that may be induced by the scanner’s hardware. In comparison to reported scBAT FF changes in response to cold exposure in literature (−1.6%7, -2.9%9), we found relatively small (~ -0.5%) and slowly changing FF dynamics in response to mild-cooling. This is likely caused by differences in the cooling procedure, such as cooling garments, cooling strength and duration. The temporal resolution may therefore be increased or decreased since the temporal evolution of scBAT FF dynamics may differ per stimulus.Conclusion
We showed the feasibility of obtaining 1-minute-resolution data of scBAT using breath-holds during cooling in healthy subjects and we demonstrated the validity and feasibility of our MRI protocol.Acknowledgements
No acknowledgement found.References
- Berbeé JFP, Boon MR, Khedoe PPSJ, et al. Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat Commun. 2015;6. doi:10.1038/ncomms7356
- Becher T, Palanisamy S, Kramer DJ, et al. Brown adipose tissue is associated with cardiometabolic health. Nat Med. 2021;27(1):58-65. doi:10.1038/s41591-020-1126-7
- Reber J, Willershäuser M, Karlas A, et al. Non-invasive Measurement of Brown Fat Metabolism Based on Optoacoustic Imaging of Hemoglobin Gradients. Cell Metab. 2018;27(3):689-701.e4. doi:10.1016/j.cmet.2018.02.002
- Cypess AM, Kahn CR. Brown fat as a therapy for obesity and diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17(2):143-149. doi:10.1097/MED.0b013e328337a81f
- Ouellet V, Labbé SM, Blondin DP, et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest. 2012;122(2):545-552. doi:10.1172/JCI60433
- Karampinos DC, Weidlich D, Wu M, Hu HH, Franz D. Techniques and Applications of Magnetic Resonance Imaging for Studying Brown Adipose Tissue Morphometry and Function. Handb Exp Pharmacol. 2019;251:299-324. doi:10.1007/164_2018_158
- Oreskovich S, Ong F, Ahmed B, et al. Magnetic resonance imaging reveals human brown adipose tissue is rapidly activated in response to cold. J Endocr Soc. October 2019. doi:10.1210/js.2019-00309
- Gashi G, Madoerin P, Maushart CI, et al. MRI characteristics of supraclavicular brown adipose tissue in relation to cold-induced thermogenesis in healthy human adults. J Magn Reson Imaging. 2019;50(4):1160-1168. doi:10.1002/jmri.26733
- Stahl V, Maier F, Freitag MT, et al. In vivo assessment of cold stimulation effects on the fat fraction of brown adipose tissue using DIXON MRI. J Magn Reson Imaging. 2017;45(2):369-380. doi:10.1002/jmri.25364
- Lundström E, Strand R, Johansson L, Bergsten P, Ahlström H, Kullberg J. Magnetic resonance imaging cooling-reheating protocol indicates decreased fat fraction via lipid consumption in suspected brown adipose tissue. PLoS One. 2015;10(4). doi:10.1371/journal.pone.0126705
- Coolbaugh CL, Damon BM, Bush EC, Welch EB, Towse TF. Cold exposure induces dynamic, heterogeneous alterations in human brown adipose tissue lipid content. Sci Rep. 2019;9(1):13600. doi:10.1038/s41598-019-49936-x
- Klein S, Staring M, Murphy K, Viergever MA, Pluim JPW. Elastix: A toolbox for intensity-based medical image registration. IEEE Trans Med Imaging. 2010;29(1):196-205. doi:10.1109/TMI.2009.2035616
Figures
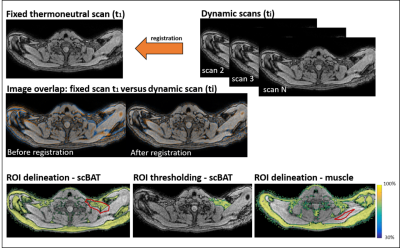
The
first thermoneutral scan was used as a reference image, and all subsequent
dynamic scans were co-registered to the first scan. First echo magnitude images
were used for co-registration and the resulting transformation matrix was used
to deform the FF map of each dynamic to match the image coordinates of the
first thermoneutral scan. ROIs were only drawn on the first thermoneutral scan
and voxels below 30% fat were excluded in both fixed and dynamic scans to avoid
inclusion of non-fatty tissue for scBAT, whereas no thresholds were used for
muscle.
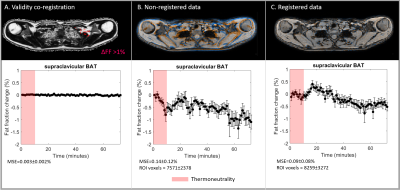
Fat
fraction changes (%) for the registration validation experiment (A), non-registered data
(B) and registered data (C). scBAT FF changes above 1% are shown in red in (A).
Image overlap between the first thermoneutral (reference) scan and the last
dynamic are shown for B and C, where the reference scan and the last dynamic
are shown in blue and orange. Red denotes thermoneutrality. The mean squared
error and the number of voxels in the scBAT volume are reported.
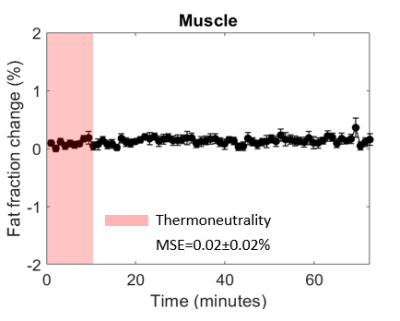
Fat
fraction differences plotted as a function of time for the control tissue: the trapezius muscle. Skeletal muscle FF differences were determined from registered data. The red-shaded area indicates the thermoneutral period and
the mean squared error is reported.
DOI: https://doi.org/10.58530/2022/2161