2159
Infant Brain Segmentation based on Multi-dMRI contrast Attention (BISMA)1Department of Radiology, Children's Hospital of Philadelphia, Philadelphia, PA, United States, 2Department of Bioengineering, University of Pennsylvania, Philadelphia, PA, United States, 3Shandong University Cheeloo College of Medicine, Jinan, China, 4Department of Radiology, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
On widely used relaxation-based T1-weighted (T1w) and T2-weighted (T2w) MRI images, 0-2-year-old infant brain images exhibit poor gray and white matter contrasts due to dynamic and poor myelination in this stage. Such poor T1w and T2w contrasts hinder accurate segmentation of infant brains based on T1w or T2w images. Instead, diffusion MRI (dMRI)-derived maps offer high contrasts throughout infancy. To leverage rich dMRI contrasts, we established an Infant Brain Segmentation based on Multi-dMRI contrast Attention (BISMA) technique. BISMA fills the gap in accurate deep learning segmentation of infant brain by fusing multi-contrasts of dMRI-derived maps.
Purpose
On most widely used traditional relaxation-based T1-weighted (T1w) and T2-weighted (T2w) MRI images, 0-2-year-old infant brain images exhibit large within-tissue intensity variations, and regionally heterogenous and dynamic contrast changes due to the ongoing myelination process in infant brains1, resulting in poor contrast among brain gray matter (GM), white matter (WM), and cerebral spinal fluid. Existing segmentation algorithms relying on relaxation-based contrasts consequently achieve relatively low accuracy2 (Fig. 1). Diffusion MRI (dMRI)-derived maps offer high inter-tissue contrasts and high intra-tissue consistency throughout infant brain development due to their robustness to the myelination process3-5. In this study, we establish an Infant Brain Segmentation based on Multi-dMRI contrast Attention (BISMA) technique to leverage the rich information from dMRI contrasts. BISMA fills the gap in deep learning (DL) segmentation of infant brain fusing multi-contrasts of dMRI maps.Methods
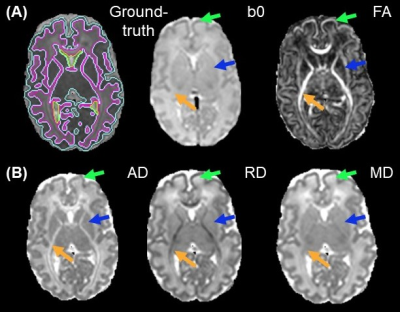
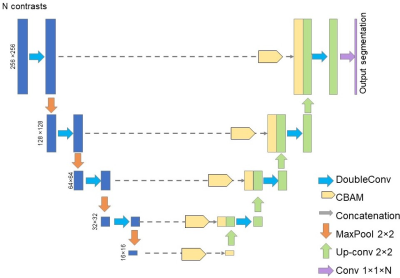
Acquisition of structural and diffusion MRI: With approval from the institutional review board, a cohort of 89 neonates with dMRI and T2w images was acquired. DMRI was at 1.5×1.5×1.6mm3 resolution with b=1000 and 1600 s/mm2, 30 gradient directions for each b-value. T2w was acquired with the same resolution. Diffusion tensor imaging (DTI) fitting: After correction of eddy current distortion and affine registration of diffusion weighted images, DTI metrics were fitted. Fractional anisotropy (FA), axial (AD), radial (RD) and mean diffusivity (MD) maps were obtained (Fig. 2A-2B). Manual segmentation for ground-truth: Manual segmentation of the datasets from 14 neonates based on DTI-derived maps was done by an experienced neuroanatomist (Fig. 2A, left). Segmentation on single contrast: A UNet model6 was trained to segment neonate brains using single dMRI contrast, with 13 subjects used for training and 1 subject leaved for validation. The network depth was 5. Starting learning rate was 0.01. StepLR scheduler with momentum=0.9 and stochastic gradient descent (SDG) optimizer was used. The network was trained for 10 epochs. Segmentation by BISMA with AD and RD contrasts: We adopted the convolutional bottleneck attention module (CBAM)7 into a UNet architecture (Fig. 3) to form the BISMA model. At each level of the UNet, CBAM is applied to the extracted feature maps. The BISMA model was trained using the same parameters as UNet for single contrast.Results
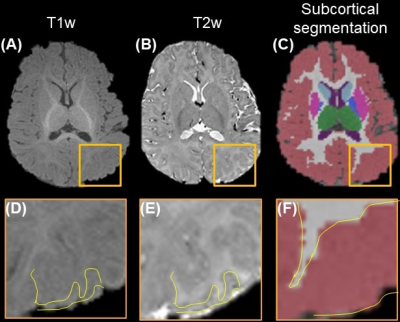
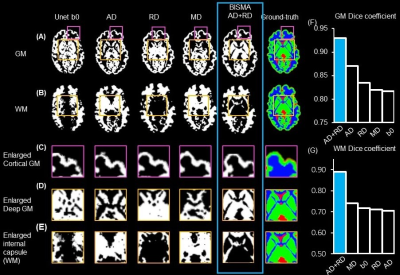
For 3-6-month-old infants, segmentation based on T1w and T2w MRI usually suffers from low accuracy due to the poor contrasts (Fig. 1A-1B). Yellow contour on enlarged T1w (Fig. 1D), and T2w (Fig. 1E) show the ground-truth segmentation, whereas yellow contour in Fig. 1F shows the T1w-based segmentation done by Infant Freesurfer2 deviating from the ground-truth. DTI contrasts offer rich complementary information about the underlying tissue types (Fig. 2). Fig. 2A shows the ground-truth segmentation along with b0 and FA contrasts on a representative neonate subject. RD and MD offer superior contrast for cortical GM (green arrows), where as AD offers the best contrast for deep GM (blue arrows). FA outperforms other contrasts for showing myelinated WM (yellow arrows). UNet6 segmentations of GM (Fig. 4A) and WM (Fig. 4B) on single contrast along with segmentation from BISMA combining AD and RD contrasts exhibit differential regional accuracies (Fig. 4A-4B) compared with ground-truth (Fig. 4A-B right-most column). Enlarged cortical GM segmentation (Fig. 4C) demonstrate superior performance of BISMA in comparison to that from any single contrast. Enlarged deep GM (Fig. 4D) also demonstrates superior performance of BIMSA in segmenting deep GM, where AD alone under-estimates and RD alone overestimates these areas.Similarly, Fig. 4E demonstrates the performance of BISMA in delineating internal capsule in WM in comparison to single contrasts. BISMA with AD+RD as inputs improves overall GM Dice by 6% (Fig. 4F) to 0.93. WM BISMA Dice coefficient reaches 0.89, improved by >10% compared to single contrasts (Fig. 4G). All Dice improvements are larger than common inter-rater segmentation error of 5% for neonate brain MRI8.
Discussion and Conclusion
Our results show single DTI contrasts’ sensitivity in separating WM and GM for neonates in comparison to conventional relaxation-based contrasts, along with the benefits of fusing multiple contrasts with BISMA. DMRI-derived contrasts depend mainly on micrometer level cellular tissue properties that remain sensitive to tissue differences despite poor infant brain myelination3-5. Different DTI-derived maps offer complementary information about the underlying tissue types, and such contrasts perform better in segmenting GM and WM in neonate brain compared with non-diffusion weighted image. Segmentation based on single contrast tends to overestimate or under-estimate the GM or WM segmentation, whereas BISMA resolves the over- or under-estimation with segmentations close to ground-truth. Moreover, fusing AD and RD contrasts with BIMSA improves the Dice for segmenting both GM and WM for more than 5%, larger than common inter-rater segmentation error for neonate brain MRI7. BISMA will be extended to full infancy age range of 0-2 years old and incorporating diffusion kurtosis imaging (DKI)9 or neurite orientation dispersion and density imaging (NODDI)10 contrasts that may further improve the segmentation accuracy.Acknowledgements
This study is funded by NIH MH092535, MH092535-S1 and HD086984.References
1. Li G, Wang L, Yap PT, Wang F, Wu Z, Meng Y., …, Shen D. Computational neuroanatomy of baby brains: A review. NeuroImage, 2019;185: 906-925.
2. Zöllei L, Iglesias JE, Ou Y, Grant PE, Fischl B. Infant FreeSurfer: An automated segmentation and surface extraction pipeline for T1-weighted neuroimaging data of infants 0–2 years. Neuroimage, 2020; 218: 116946.
3. Huang H, Shu N, Mishra V, Jeon T, Chalak L, Wang ZJ, ..., He Y. Development of human brain structural networks through infancy and childhood. Cerebral Cortex. 2015; 25.5:1389-1404.
4. Huang H, Zhang J, Wakana S, Zhang W, Ren T, Richards LJ, ..., Mori S. White and gray matter development in human fetal, newborn and pediatric brains. Neuroimage. 2006; 33.1: 27-38.
5. Ouyang M, Dubois J, Yu Q, Mukherjee P, Huang H. Delineation of early brain development from fetuses to infants with diffusion MRI and beyond. Neuroimage. 2019;185: 836-850
6. Ronneberger O, Fischer P, Brox T. U-net: Convolutional networks for biomedical image segmentation. MICCAI. 2015; pp. 234-241. Springer, Cham.
7. Woo S, Park J, Lee JY, Kweon IS. Cbam: Convolutional block attention module. Proceedings of the European conference on computer vision (ECCV). 2018; 3-19.
8. Weisenfeld NI, Warfield SK. Automatic segmentation of newborn brain MRI. Neuroimage, 2009; 47.2: 564-572.
9. Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: the quantification of non‐gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med: 2005; 53.6: 1432-1440.
10. Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012; 61.4: 1000-1016.
Figures