2158
Efficient, streamline-free white matter tract segmentation for intraoperative diffusion MRI.1UCL Great Ormond Street Institute of Child Health, University College London, London, United Kingdom, 2Department of Radiology, Great Ormond Street Hospital for Children, London, United Kingdom, 3Lysholm Department of Neuroradiology, The National Hospital for Neurology & Neurosurgery, University College London Hospitals NHS Foundation Trust, London, United Kingdom, 4Neuroradiological Academic Unit, Department of Brain Repair and Rehabilitation, UCL Queen Square Institute of Neurology, London, United Kingdom, 5Department of Neurosurgery, Great Ormond Street Hospital for Children, London, United Kingdom
Synopsis
Tractfinder is an atlas-based approach to segmenting white matter tracts without tractography, in subjects with space-occupying lesions. A shape and orientation prior is combined with fibre orientation distributions in the target data to produce a map of tract location. Results for a given tract can be obtained in a matter of minutes and are comparable to those afforded by clinical tractography. In future, tractfinder could be applied to intraoperative diffusion MRI to aid neuronavigation during brain surgery.
Introduction
An understanding of the spatial relationship between tumour and adjacent intact white matter is critical to safe resection of brain tumours. Although relevant tracts can be defined preoperatively from diffusion MR imaging (dMRI), their relationships change once brain shift has occurred during resection.1,2 Intraoperative dMRI offers a potential solution for identifying tracts during surgery.Streamline tractography is the current clinical standard for reconstructing white matter tracts preoperatively. However, implementation can be time-consuming, and obtaining accurate results in the presence of tumours and oedema is difficult.3,4 While the availability of time, computational resources and expert operators can mitigate these problems offline, any intraoperative image processing needs to be rapid, largely automated, and accurate.
We present initial results for a tractography-free white matter segmentation method ("tractfinder"), developed specifically for the surgical context. The proposed atlas-based method broadly comprises three components: a tract-specific orientation and location atlas, a tumour deformation model, and a spherical harmonic based operation to combine the atlas and (intraoperative) data.
Methods
A tract atlas was created by combining carefully selected tractography streamlines from 16 healthy subjects in MNI152 template space.5 Once each subject's reconstruction was linearly registered to template space, a tract orientation distribution (TOD) map6 was derived from the streamlines and normalised to remove streamline density information, leaving only orientation information. The normalised TOD maps were then averaged across all subjects, creating a tract-specific TOD atlas where the TOD magnitude in each voxel is the proportion of training subjects in which the tract was present there, up to a scaling constant.To segment a tract, the relevant TOD atlas is linearly registered from template into subject space. If a tumour is present, then a deformation field is calculated from the subject's brain and manually segmented tumour volumes, using a radial expansion model adapted from Nowinski and Belov (2005),7 and applied to the tract atlas (Figure 1).
Fibre orientation distribution (FOD) reconstruction is performed in the target subject using constrained spherical deconvolution (CSD),8 after dMRI data preprocessing (including denoising and eddy current distortion correction).9,10,11,12
To calculate the agreement between atlas and data, the atlas TOD and subject FOD in each voxel (both represented in the spherical harmonic (SH) basis) are multiplied and integrated over the sphere in a single operation amounting to the inner product of their SH coefficients (the intermediate step of distribution multiplication is displayed in Figure 2, "Multiplied ODFs", for illustration only).
Tract atlases have been created for the corticospinal tract (CST) and optic radiation (OR), two tracts of frequent interest in neurosurgery. Tractfinder was evaluated on healthy control data, preoperative paediatric tumour data and on adult pre- and intraoperative tumour data.
Results and Discussion
Results were compared with probabilistic streamline tractography, using MRtrix3 tools,9,13 and TractSeg with default options.14 TractSeg was chosen for comparison as it is a modern, tractography-free tract segmentation approach that performs well in healthy data. It is comparable to the proposed approach in execution timescale and in that it operates on CSD-derived FODs and has also been proposed for use in the context of surgical planning for brain tumour resection.15 By contrast, TractSeg is a deep learning approach and doesn't incorporate any explicit handling of tumour deformations.Processing time (including preprocessing, CSD reconstruction, tumour deformation modelling and tractfinder, but excluding registration) totalled an average of 5 minutes for 5 different intraoperative MRI datasets, compared with 15-25 minutes for region of interest placement and tractography alone, and 3-5 minutes for TractSeg.
Sample results for healthy and tumour data are shown in Figures 3-5. In the absence of a ground truth, a quantitative validation is unobtainable, however overall, there is good agreement between tractfinder and tractography, the current clinical standard. In certain difficult to track areas with crossing fibres, which are often excluded from tractography and TractSeg reconstructions, tractfinder is able to pick out the relevant FOD peaks and find evidence for the tract, albeit with slightly reduced signal (Figure 2).
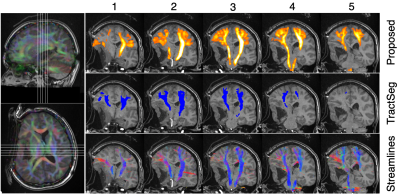
Figures 4 and 5 show tractfinder results for pre- and intraoperative MRI scans of an adult patient who underwent resection of an epidermoid cyst. The preoperative CST map successfully captures the tract's displacement and sharp bend around the cyst (Figure 4). For the intraoperative data, tumour deformation was repeated using the preoperative cyst segmentation scaled down to 80% to account for partial resection, thus avoiding the need for intraoperative cyst segmentation. The resulting intraoperative CST reconstruction agrees well with tractography results (Figure 5).
Conclusion
We have demonstrated initial findings for a simple and effective atlas-based white matter segmentation method which has been developed specifically for tumour cases and for application in a neurosurgical context.Tractfinder produces results qualitatively at least as good as streamline tractography, which is the current clinical standard for reconstructing white matter tracts. It demonstrates better sensitivity than tractography in some difficult to track areas, while atlas adjustments to account for tumour deformation enable effective detections of displaced tracts.
These results are a promising step towards an automated tract segmentation method that could be integrated into an intraoperative imaging pipeline to substantially improve intraoperative neuronavigation and thus neurosurgical outcomes.
Acknowledgements
This work is supported by the EPSRC-funded UCL Centre for Doctoral Training in Intelligent, Integrated Imaging in Healthcare (i4health) (EP/S021930/1) and the Department of Health’s NIHR-funded Biomedical Research Centre at Great Ormond Street Hospitals.References
- Nimsky, C. et al. Quantification of, Visualization of, and Compensation for Brain Shift Using Intraoperative Magnetic Resonance Imaging. Neurosurgery 47, 1070–1080 (2000).
- Nimsky, C. et al. Intraoperative Diffusion-Tensor MR Imaging: Shifting of White Matter Tracts during Neurosurgical Procedures—Initial Experience. Radiology 234, 218–225 (2005).
- Toescu, S. M., Hales, P. W., Tisdall, M. M., Aquilina, K. & Clark, C. A. Neurosurgical applications of tractography in the UK. Br. J. Neurosurg. 1–6 (2020) doi:10.1080/02688697.2020.1849542.
- Nimsky, C. Fiber Tracking—A Reliable Tool for Neurosurgery? World Neurosurg. 74, 105–106 (2010).
- Fonov, V., Evans, A., McKinstry, R., Almli, C. & Collins, D. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. Neuroimage 47, S102 (2009).
- Dhollander, T. et al. Track orientation density imaging (TODI) and track orientation distribution (TOD) based tractography. Neuroimage 94, 312–336 (2014).
- Nowinski, W. L., & Belov, D. (2005). Toward atlas-assisted automatic interpretation of MRI morphological brain scans in the presence of tumor. Academic Radiology, 12(8), 1049–1057. https://doi.org/10.1016/j.acra.2005.04.018
- Tournier, J. D., Calamante, F. & Connelly, A. Robust determination of the fibre orientation distribution in diffusion MRI: Non-negativity constrained super-resolved spherical deconvolution. Neuroimage 35, 1459–1472 (2007).
- Tournier, J.-D. et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage 202, 116137 (2019).
- Andersson, J. L. R. & Sotiropoulos, S. N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 125, 1063–1078 (2016).
- Smith, S. M. et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, S208–S219 (2004).
- Veraart, J. et al. Denoising of diffusion MRI using random matrix theory. Neuroimage 142, 394–406 (2016).
- Tournier, J.-D. & , F. Calamante, and a. C. Improved probabilistic streamlines tractography by 2nd order integration over fibre orientation distributions. in Proceedings of the International Society for Magnetic Resonance in Medicine vol. 18 1670 (2010).
- Wasserthal, J., Neher, P. & Maier-Hein, K. H. TractSeg - Fast and accurate white matter tract segmentation. Neuroimage 183, 239–253 (2018).
- Güllmar, D. et al. Feasibility study on automated white matter tract segmentation in neurosurgical preoperative planning. in Proceedings of the International Society for Magnetic Resonance in Medicine vol. 27 0895 (2019).
Figures
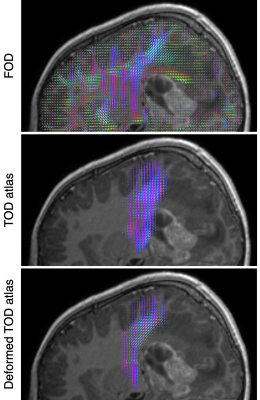
Figure 1 Tumour
deformation demonstrated in a paediatric patient (11y, F) with a grade 3
ependymoma. Top: Fibre orientation distributions from patient dMRI
data. Middle: linearly registered CST TOD atlas. Bottom: after tumour
deformation modelling, the atlas displays closer positional and
orientational agreement with the data.
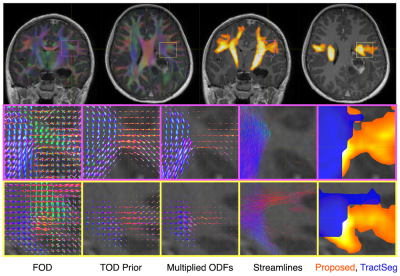
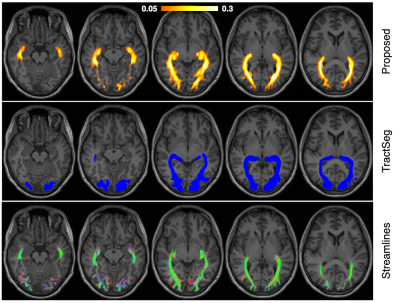
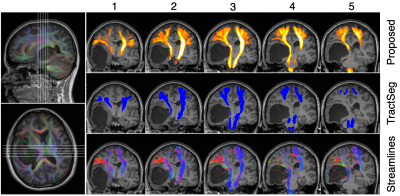
Figure 3 Tractfinder output for the optic radiation in a healthy adult subject (top). A colourbar is given to indicate relative confidence but the scaling is arbitrary. The entire tract is well reconstructed and corresponds well with tractography (bottom), including extensive inclusion of Meyers Loop (the anterior extension of the tract), a region that TractSeg (middle) struggles with by comparison.