2155
Automated brain morphometry for sub-millimeter 7T MRI using transfer learning1Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 2Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 3LTS5, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 4Siemens Healthcare Pvt. Ltd., Bangalore, India, 5Support Center for Advanced Neuroimaging, Institute for Diagnostic and Interventional Neuroradiology, Inselspital, Bern University, Bern, Switzerland, 6Translational Imaging Center, sitem-insel AG, Bern, Switzerland, 7Advanced Clinical Imaging Technology, Siemens Healthcare AG, Bern, Switzerland, 8Magnetic Resonance Methodology, Institute of Diagnostic and Interventional Neuroradiology, University of Bern, Bern, Switzerland, 9Department of Neuroradiology, Medical Center – University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany
Synopsis
Large spatial signal variations due to field inhomogeneities complicate the application of automated brain morphometry at 7T. In this work, we propose to use transfer learning to adapt a template-based segmentation algorithm to sub-millimeter ultra-high field applications. More specifically, a convolutional neural network pre-trained on T1-weighted scans to extract the total intracranial volume (TIV) from MP-RAGE acquisitions was re-trained to retrieve the TIV mask directly from MP2RAGE volumes. The developed method proved to reliably deliver brain tissue masks and volumetry at 7T.
Introduction
Automated brain morphometry from MR images has been shown to deliver promising neuroimaging biomarkers to monitor structural changes during aging and the progression of neurodegenerative disease. Most of the tools available for automatically analyzing structural brain MR images has been optimized for 3D T1-weighted contrasts1–3, with the MP-RAGE being the most commonly used sequence at 1.5T and 3T4. For clinical applications at 7T, the MP2RAGE sequence is preferred as it provides a bias-free 3D T1-weighted image (“UNI”)5. However, the salt-and-pepper noise surrounding brain tissues in the UNI images affects automated skull-stripping methods, often resulting in inaccurate masks of the total intracranial volume (TIV). A solution to this particular problem has been proposed by deriving a MP-RAGE-like image (“combT1w”) through multiplying the MP2RAGE UNI and GRE2 volumes6. However, at 7T, this solution is not ideal since the GRE2 suffers from large spatial signal variations due to B1+ and B1- inhomogeneities that are difficult to correct and may result in wrong segmentations. Alternatively, a simple modification to the MP2RAGE normalized complex ratio was also proposed to suppress the noise in the UNI image7. However, such modification requires the tuning of a correction factor which is impractical in a clinical scenario7.In this work, we propose a new pipeline for automated brain morphometry at 7T from MP2RAGE acquisitions using an in-house built prototype software, MorphoBox3, combined with a convolutional neural network (CNN) that performs brain skull-stripping directly on the UNI image. The developed pipeline was compared and validated against the original algorithm both at 3T and 7T.
Methods
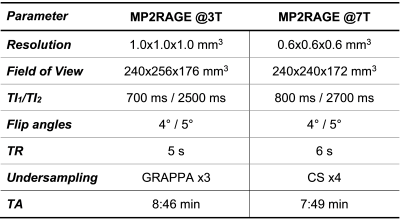
Study population and MR protocolTwo cohorts of healthy individuals were scanned at two different field strengths:
- 201$$$\,$$$subjects (123$$$\,$$$females, age$$$\,$$$=$$$\,$$$[20-64]$$$\,$$$y/o) at 3T (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany);
- 19$$$\,$$$subjects (11$$$\,$$$females, age$$$\,$$$=$$$\,$$$[15-72]$$$\,$$$y/o) at 7T (MAGNETOM Terra, Siemens Healthcare, Erlangen, Germany).
Brain segmentation
The template-based method used by MorphoBox for segmenting the TIV was replaced by a CNN-based algorithm recently proposed by Venkategowda et al.8. The CNN was trained on more than 400 T1-weighted scans to extract the TIV from MP-RAGE acquisitions8. Here, a transfer learning strategy was employed to achieve the same goal for MP2RAGE UNI data. More specifically, the CNN algorithm pre-trained on MP-RAGE data was re-trained to retrieve the TIV mask directly from UNI volumes. To that end, ground truth masks were obtained by segmenting MP2RAGE 3T data with the original pipeline of MorphoBox on the respective combT1w image. The network was re-trained for 200 epochs using the Dice coefficient between the predicted and target masks as loss function. Images from 181 of the subjects scanned at 3T were used for the training.
After extracting the TIV, brain volumes were segmented on the UNI images using the original MorphoBox pipeline3.
Validation
Volumes of brain regions estimated with the original pipeline (i.e., TIV extraction on the combT1w) and the new proposed solution (i.e., TIV extraction directly on the UNI) were computed in the remaining 20 testing datasets acquired at 3T and compared via Wilcoxon rank-sum tests. P-values were corrected for multiple comparisons with the Benjamini-Hochberg procedure.
Segmentation masks produced by the original and new pipeline applied to the 7T data were visually compared. Masks obtained in patients scanned at both field strengths were also visually compared.
Results
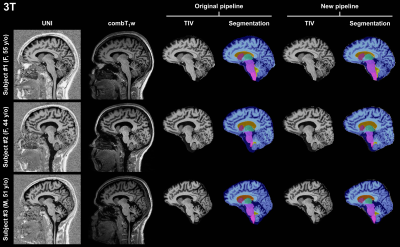
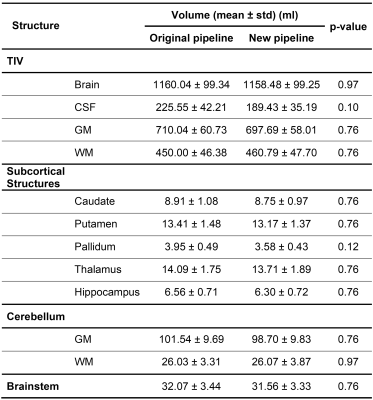
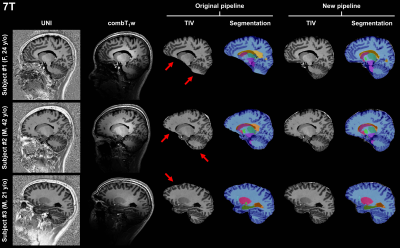
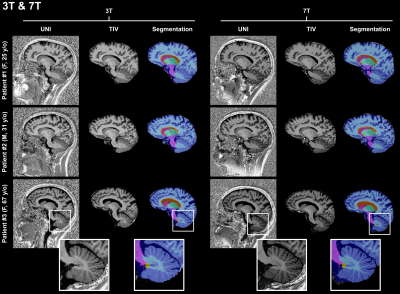
Representative sagittal slices of the retrieved segmentation masks are shown in Figure$$$\,$$$1 for three subjects scanned at 3T. At visual inspection, the new pipeline delivered reliable masks that are comparable to the original pipeline. Quantitatively, no significant volume differences were found in any of the segmented brain regions when comparing the two pipelines (see Table$$$\,$$$2).Segmentation masks obtained from 7T data acquired in healthy subjects are shown in Figure$$$\,$$$2. In this case, TIV masks are wrongly estimated by the original pipeline, especially in regions with large intensity variations in the combT1w contrast (e.g., frontal lobe, temporal lobe, and cerebellum). Conversely, TIV masks were correctly segmented with the proposed pipeline.
In comparison to segmentation masks obtained at 3T in the same patient, masks obtained from 7T data visually appear to provide a better definition of tissue boundaries and small structures, as for instance the cerebellum (see Figure$$$\,$$$3).
Discussion and Conclusion
This work introduced a new method for automated brain morphometry at 7T. Using transfer learning from a CNN algorithm pre-trained on 3T MP-RAGE data, TIV masks were directly retrieved from 7T MP2RAGE UNI data. The proposed strategy was quantitatively validated by comparing the estimated volumes with those segmented from the original pipeline at 3T showing no significant differences. Future work should focus on validating the developed pipeline in a larger cohort of patients. Additionally, the benefit of performing MP2RAGE acquisitions in combination with parallel transmission at 7T should be investigated as it allows to reduce spatial signal variations.The higher resolution of 7T MRI combined with the proposed automated morphometry may enable more accurate and reproducible monitoring of brain volumes both in cross-sectional and longitudinal studies.
Acknowledgements
No acknowledgement found.References
1. Ashburner J. Computational anatomy with the SPM software. Magn Reson Imaging. 2009;27(8):1163-1174. doi:10.1016/j.mri.2009.01.006
2. Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774-781. doi:10.1016/j.neuroimage.2012.01.021
3. Schmitter D, Roche A, Maréchal B, et al. An evaluation of volume-based morphometry for prediction of mild cognitive impairment and Alzheimer’s disease. NeuroImage Clin. 2015;7:7-17. doi:10.1016/j.nicl.2014.11.001
4. Mugler JP, Brookeman JR. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE). Magn Reson Med. 1990;15(1):152-157. doi:10.1002/mrm.1910150117
5. Marques JP, Kober T, Krueger G, van der Zwaag W, Van de Moortele P-F, Gruetter R. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage. 2010;49(2):1271-1281. doi:10.1016/j.neuroimage.2009.10.002
6. Fujimoto K, Polimeni JR, van der Kouwe AJW, et al. Quantitative comparison of cortical surface reconstructions from MP2RAGE and multi-echo MPRAGE data at 3 and 7T. Neuroimage. 2014;90:60-73. doi:10.1016/j.neuroimage.2013.12.012
7. O’Brien KR, Kober T, Hagmann P, et al. Robust T1-weighted structural brain imaging and morphometry at 7T using MP2RAGE. PLoS One. 2014;9(6). doi:10.1371/journal.pone.0099676
8. Venkategowda PB, Kumaraswamy AK, Richiardi J, et al. Retrofitting a Brain Segmentation Algorithm with Deep Learning Techniques: Validation and Experiments. In: Proceedings of the International Society of Magnetic Resonance Imaging. 2020. Abstract number: 3566.
Figures