2152
Investigation of heating risks during MR-guided microwave ablation using applicators without active shaft cooling1Radiology, Mayo Clinic, Rochester, MN, United States
Synopsis
Execution of MR sequences (RF-heating) and/or microwave energy during MRgMWA may lead to harmful heating at tissue surface with non-cooled MR-conditional applicators. MR imaging was performed using a high SAR sequence and several geometric orientations of phantom/applicators to assess RF-heating impact. Applicator insertion depth (with/without ultrasound gel) and spacing of two applicators on shaft heating were evaluated. Negligible temperature changes due to RF-heating were observed. Significant temperature increases were measured during MWA due to action of MWA system/applicator, which can be mitigated by increasing the applicator insertion depth. Further reduction in temperature change can be achieved by using ultrasound gel.
Introduction
Image-guided microwave ablation (MWA) has gained increasing interests in clinical interventional practices to treat localized tumors1-3. Despite the advantages of using magnetic resonance imaging (MRI) to guide MWA procedure including real-time MR thermometry, availability of MRI-conditional MWA systems is currently limited and MR-guided MWA (MRgMWA) has only been performed at a few institutions. We have demonstrated the feasibility of using a commercially available “MRI-configured” MW system for MRgMWA4, 5. Although the applicators without active cooling used in this system are MRI-conditional, it is unclear whether potential harmful heating at tissue surface could occur due to execution of MR sequences (RF-heating) or due to energy of the microwave field during ablation, especially when multiple MW applicators are used. This work aims to investigate these potential safety concerns and evaluate the potential using ultrasound gel applied at insertion site to mitigate the skin burn risk.Methods
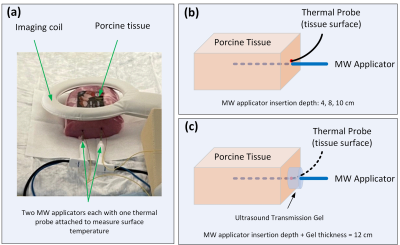
Ex-vivo porcine tissue experiments of MRgMWA were conducted on a 1.5T scanner (Ingenia, Philips, Best, Netherlands). Two separate MWA systems each consisting of a microwave generator and a large MRI-conditional applicator without active cooling were used (Avecure, MedWaves Inc., San Diego, CA)4-6. The microwave generators were connected to the applicators in the scanner room via waveguide. The Avecure system attempts to minimize the risk of tissue charring and overheating along the shaft while achieving optimal ablation performance by using relatively low power (36W), limiting the maximal temperature allowed (60-130°C), and automatically adjusting the output frequency (902-928MHz) and generator duty cycle.The experimental setup is shown in Figure 1. Unless otherwise mentioned, the microwave applicators were inserted into the tissue phantom at a depth of 10 cm (Figure 1a). Neoptix fiberoptic temperature sensors (Qualitrol, Fairport, USA) were attached to the applicators to measure the temperature change at the tissue surface continuously.
To assess RF-heating in the applicators, MR imaging was performed using a high specific absorption ratio (SAR) sequence (Turbo Spin Echo, TR/TE=70ms/971ms, ETL: 22, flip angle: 90°, constant refocusing flip angle: 100°, scan time: 2minutes and 20seconds, estimated SAR: 2.1W/kg). The experiments were repeated with the porcine tissue phantom and the two applicators positioned at several geometric orientations inside the scanner bore, including the tissue sample placed near the edge of the scanner bore and the applicators oriented at roughly 45° angle to the horizontal plane and either parallel or perpendicular to the side of the bore7. Different combinations of applicator status such as whether they were connected to the microwave generators and whether the generators were powered off and in standby mode were tested.
To investigate impact of applicator insertion depth and spacing of the applicators on shaft heating, two sets of experiments were performed: one with a single applicator and one with two applicators. In the set of experiments with a single applicator, six ablations were performed with different applicator insertion depth into the tissue with/without a cylindrical layer of ultrasound gel surrounding the applicator at the insertion site: 4cm insertion depth with/without 8cm thick layer of gel, 8cm insertion depth with/without 4cm thick layer of gel, 10cm insertion depth with/without 2cm thick layer of gel. The cylindrical layer diameter was about 2cm. The effective insertion depth of the applicators inside both tissue and ultrasound gel was therefore maintained at ~12cm. In the set of experiments with two applicators, three experiments were performed with the applicators inserted in parallel at 2.6, 3.6 and 4.6cm apart, respectively. The ablation duration was 10minutes for all these experiments unless otherwise indicated and each individual MWA procedure was performed on a separate phantom of fresh tissue to provide an identical baseline of tissue condition.
Results
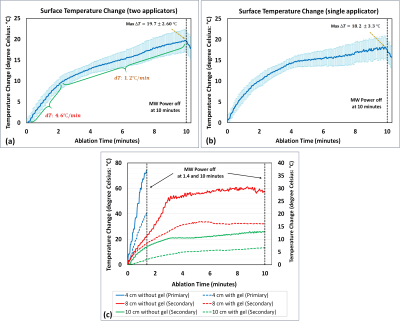
No significant RF-heating at tissue-air interfaces was found in any applicator orientation investigated. Figure 2 shows measured temperature increases resulting from the action of MWA system in case of a single applicator (Figure 2a) and two simultaneous applicators (Figure 2b), which were similar in both scenarios. Temperature increases at the tissue surface were measured as 73℃,31℃,and 14℃ (without gel) and 40℃,16℃,and 6℃ (with gel) for MWA with the applicator insertion depth of 4, 8, and 10cm in the tissue phantom (Figure 2c).Discussion
The temperature increases at the tissue surface were significantly decreased with deeper applicator insertion depth. Therefore, a minimum insertion depth (e.g., 10cm) should be considered during clinical treatments using this type of applicators. Moreover, our experiments demonstrated that application of ultrasound gel at the applicator insertion site could potentially reduce the tissue surface temperature increase by a half. During clinical ablations, presence of perfusion enhanced heat dissipation should further reduce risks of patient injury. The measured maximum surface temperature rises for one-applicator and two-applicator scenarios were not significantly different, indicating that introducing an additional applicator at various distances from the existing one would not increase the shaft heating risk.Conclusion
Negligible temperature changes due to RF-heating were observed using the MWA system. Significant temperature increases were measured during MWA due to action of MWA system/applicator, which can be mitigated by increasing the applicator insertion depth. Further reduction in temperature change can potentially be achieved by using ultrasound gel. With these precautions, MWA with two applicators can be safely performed with the system under investigation.Acknowledgements
No acknowledgement found.References
1. Chen, J.C., et al., Prostate Cancer: MR Imaging and Thermometry during Microwave Thermal Ablation-Initial Experience. Radiology, 2000. 214(1): p. 290-297.
2. Morikawa, S., et al., MR-guided microwave thermocoagulation therapy of liver tumors: Initial clinical experiences using a 0.5 T open MR system. Journal of Magnetic Resonance Imaging, 2002. 16(5): p. 576-583.
3. Kaltenbach, B., et al., Real-time qualitative MR monitoring of microwave ablation in ex vivo livers. International Journal of Hyperthermia, 2016. 32(7): p. 757-764.
4. Gorny, K.R., et al., Practical implementation of robust MR-thermometry during clinical MR-guided microwave ablations in the liver at 1.5 T. Physica Medica, 2019. 67: p. 91-99.
5. Lu, A., et al., Improved MR-thermometry during hepatic microwave ablation by correcting for intermittent electromagnetic interference artifacts. Physica Medica, 2020. 71: p. 100-107.
6. Simon, C.J., D.E. Dupuy, and W.W. Mayo-Smith, Microwave Ablation: Principles and Applications. RadioGraphics, 2005. 25(suppl_1): p. S69-S83.
7. Ma, C., et al., Protocol for testing suitability of compact US imaging systems for use inside MRI suites, and application to one commercial US system. Biomedical Physics & Engineering Express, 2016. 2(4): p. 047003.
8. Rieke, V. and K. Butts Pauly, MR thermometry. Journal of Magnetic Resonance Imaging, 2008. 27(2): p. 376-390.
Figures