2151
Combining Proton Resonance Frequency Shift and T1-mapping Thermometry with a 3D Spiral Ultra-Short Echo Time Sequence1Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 2Department of Imaging Sciences & Innovation, Geisenger, Danville, PA, United States, 3Electrical and Computer Engineering, Brigham Young University, Provo, UT, United States, 4Radiology and Medical Imaging, University of Virginia, Charlottesville, VA, United States
Synopsis
Temperature monitoring plays an essential role in transcranial MR-guided focused ultrasound (tcMRgFUS) surgery for measuring thermal dose at the target. Unintended heating of the skull and nearby brain tissue is not currently monitored. By using a two-echo 3D spiral ultra-short echo time (UTE) sequence, we demonstrate a method that combines T1-mapping and proton resonance frequency (PRF) shift thermometry to simultaneously monitor skull and brain heating and validate it in an in vitro model.
Introduction:
MR-guided Focused Ultrasound (MRgFUS) is a minimally invasive method of treating medically-refractory essential tremor and tremor-dominated Parkinson’s disease. Acoustic waves through the skull focus on the target brain tissue causing ablation with temperatures above 60°C1, and the thermal dose is monitored using MR thermometry. However, the skull absorbs substantial energy2, which may lead to pain and damage to the skull3 and nearby brain tissue. However, skull heating lacks a direct, noninvasive monitoring method, forcing the surgeon to rely entirely on computational modeling to assess the risk.The prevailing method for thermometry in aqueous tissue, proton resonance frequency (PRF) shift, is based on a temperature-dependent phase shift, which increases with TE4. However, PRF is not practical in short-T2 tissues such as bone, so the variable flip angle (VFA) method for T1 mapping is an alternative. Han et al. quantified temperature-dependent T1 changes in cortical bone using ultra-short echo time (UTE) MRI5. Combining these two thermometry methods using a two-echo rapid UTE sequence has the potential to simultaneously monitor unintended skull and brain heating in tcMRgFUS.
In this work, we used a two-echo 3D spiral UTE sequence for thermometry with both the VFA and PRF methods. To demonstrate its feasibility, we made bone-gel phantoms and conducted experiments under heating using a water bathing and focused ultrasound (FUS).
Methods:
All experiments were performed in a 3T scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany). For the water-bath experiment, a product transmit/receive head coil was used. Parameters used for the 3D UTE sequence were: TR 30ms, TE 0.05/10ms, flip angles 15°/30°, matrix size 96x96x10, resolution 1.35x1.35x8mm3, spiral interleaves 64, RF duration 60µs, TA per FA 36s. As the cooling process was very slow, the scan was paused between temperature samples for about 20 minutes.A phantom was made in a plastic bottle with agar gel surrounding ex-vivo bovine femur with the marrow removed and kept in refrigeration before use. Three optical fibers were fixed inside the cortical bone through a drilled hole, the inner gel, and the outer gel respectively (Figure 1, left). The phantom was immersed in hot water (~65℃). The scan was not started until the thermal steady state was reached, which was based on temperature reading from an optical thermometer (L201, Rugged Monitoring, Quebec, Canada).
The VFA and PRF methods were used for the data from the short and long echoes respectively. Both methods were applied to regions in the inner and outer gel. Only the VFA method was applied to the bone. The size of each ROI was 4x4 pixels. The VFA T1 values were smoothed over four cycles for display. As the PRF data had twice as many samples as the VFA data (two flip angles), smoothing was over eight cycles for PRF.
For the FUS experiment, a product flexible surface coil was used and some parameters were altered to adapt to the hardware: TE 0.07/10ms, RF duration 100µs. The phantom was placed in a plastic cylinder with no bottom, so that the ultrasound could penetrate the gel and reach vertically the bone-gel boundary (Figure 1, right). An optical fiber was inserted in the gel with a ~2cm distance to the acoustic focus to measure temperature nearby. The scan covered three FUS sonications, each of which spanned 114s with a power of 5W, as well as the cooling periods.
In contrast to the water-bath experiment, VFA was applied only to a 3x5 pixel ROI in the bone, and PRF was applied only to a 3x5 pixel ROI in the gel. Both ROIs were selected near the boundary. Thus, it could be assumed that the temperature for the gel by PRF was approximately the same as for the bone.
Results:
In the water-bath experiment, the PRF results from the inner and outer gel were within a 1.5°C deviation from the values from the optical fiber thermometer (OFT). The linear relationship between the OFT temperatures and the T1 values of the bone by VFA could be clearly observed (Figure 2).By first-order polynomial fit over the OFT values and the T1 values in the gel, the slopes were 38.42ms/°C and 36.07ms/°C for the inner and outer gel respectively (Figure 3). The slope for the bone was 2.51ms/°C (not shown).
In the FUS experiment, the PRF results from the gel and the VFA results from the bone displayed a linear relationship. By fitting between them, a slope of 3.86ms/°C was calculated, which is comparable to the value, 2.51ms/°C, in the water-bath experiment (Figure 4).
Conclusion:
A two-echo 3D spiral UTE sequence was used for simultaneously monitoring the temperature of bone and agar gel. By the water-bath experiments, a linear relationship between the VFA T1 values and the ground truth temperatures in gel was demonstrated with very good agreement. By the FUS experiment, a linear relationship between the (assumed) ground truth temperature and the VFA T1 in bone was determined, with evidence that the slopes of the fits were comparable to each other between these two experiments. In conclusion, this is a promising method for simultaneously measuring off-target skull and brain heating in tcMRgFUS. Future work includes accelerated imaging, improving SNR, and in vivo testing.Acknowledgements
This research was partly supported by NIH R01 EB028773, the Focused Ultrasound Foundation, and Siemens Healthcare. The authors acknowledge Josef Pfeuffer, Thomas Benkert, and Berthold Kiefer for their help with this project.
References
[1] Gilbo Y, Sporkin H, Fielden S, Mugler JP, Miller W, Allen S, Meyer CH. Detecting T1-based signal reduction in focused ultrasound heating of bone using a 3D spiral ultra-short echo time sequence. Proceedings 27th Annual Meeting ISMRM, Montréal. 2019:3812.
[2] Pinton G, Aubry JF, Bossy E, Muller M, Pernot M, Tanter M. Attenuation, scattering, and absorption of ultrasound in the skull bone. Med Phys. 2012 Jan;39(1):299-307.
[3] Schwartz ML, Yeung R, Huang Y, Lipsman N, Krishna V, Jain JD, Chapman MG, Lozano AM, Hynynen K. Skull bone marrow injury caused by MR-guided focused ultrasound for cerebral functional procedures. J Neurosurg. 2018 May 4;130(3):758-762.
[4] Sporkin H, Gilbo Y, Fielden S, Mugler JP, Miller W, Pfeuffer J, Kiefer B, Meyer CH. Detecting T1-based signal reduction in focused ultrasound heating of bone at 1.5T using a 3D spiral ultra-short echo time sequence. Proceedings 26th Annual Meeting ISMRM, Paris. 2018:1495
[5] Han M, Rieke V, Scott SJ, Ozhinsky E, Salgaonkar VA, Jones PD, Larson PE, Diederich CJ, Krug R. Quantifying temperature-dependent T1 changes in cortical bone using ultrashort echo-time MRI. Magn Reson Med. 2015 Dec;74(6):1548-55.
Figures
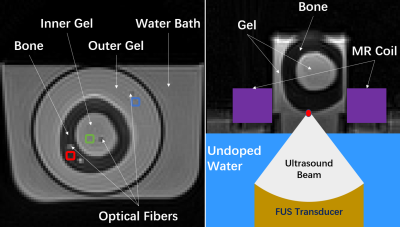
Figure 1. The cortical bone without marrow was surrounded by agar gel both inside and outside the bone. For the water-bath experiment (left), the bone-gel phantom was immersed in hot water during the cooling process. 4x4 pixel ROIs in the bone (red), the inner gel (green), and the outer gel (blue) were selected near each optical fiber. For the FUS experiment (right), the bone-gel phantom was in a plastic cylinder with no bottom. The ultrasound beam reached vertically the boundary between the bone and the gel. The ring-shaped MR coil surrounded the cylinder.
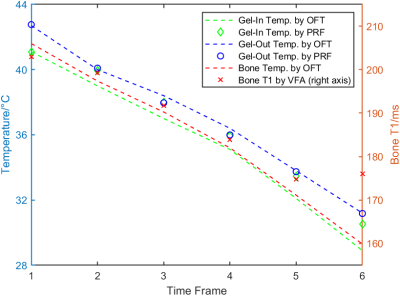
Figure 2. Water-bath experiment. Temperature measured by the optical fiber thermometer (OFT) and PRF was plotted versus T1 values calculated by VFA. All data were smoothed over four scans and averaged across the corresponding 4x4 pixel ROIs described in Figure 1.
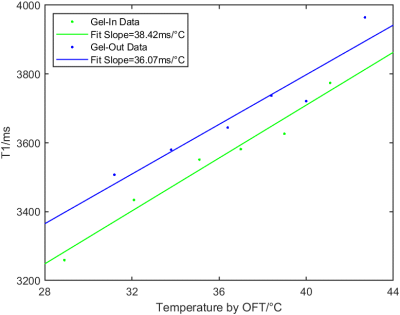
Figure 3. Water-bath experiment. The linear relation between T1 and temperature values in gel was plotted. The T1 values were calculated by VFA. The temperature values were measured by OFT. The slopes of the fits were 38.42ms/°C and 36.07ms/°C for the inner and outer gel respectively. All data were smoothed over four scans and averaged across the corresponding 4x4 pixel ROIs described in Figure 1. The slope of the fit for the bone was 2.51ms/°C (not shown).
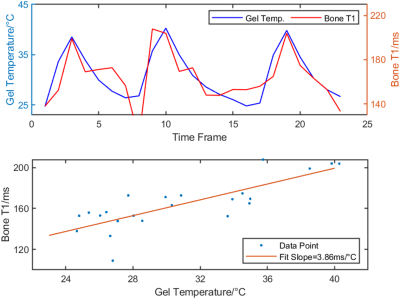
Figure 4. FUS experiment. the top figure displays the gel temperature by PRF and the bone T1 by VFA. Both data sets were averaged across a 3x5 pixel ROI selected on the boundaries of the bone and the gel. The PRF data was also averaged over every two frames to be compatible with the VFA data. The bottom figure displays the fitting result based on this data with the assumption that the bone and the gel shared the same temperature values. The slope of the fit was 3.86ms/°C.