2149
Deep learning-assisted ablative margin analysis for MR-guided prostate focal cryoablation: feasibility and initial retrospective validation1Medical Imaging, Radboudumc, Nijmegen, Netherlands, 2Robotics and Mechatronics, University of Twente, Enschede, Netherlands
Synopsis
MR-guided focal cryoablation is an emerging treatment option for localized prostate cancer, however local recurrence due to incomplete ablation is not uncommon. Ablation completeness is typically assessed on intraprocedural imaging by side-by-side comparison, but a volumetric approach is lacking. We present a deep learning-assisted algorithm for near real-time ablative margin monitoring during cryoablation procedures. Retrospective validation in 27 patients after MR-guided prostate cryoablation demonstrated significantly smaller minimal ablative margin and percentual tumour coverage for patients with versus without local recurrence. Prospective use may aid physicians in reducing the risk of local recurrence during prostate cryoablation procedures.
Introduction
MR-guided focal cryoablation is an emerging treatment option in localized primary or recurrent prostate cancer after primary radiotherapy1. Although reported technical success rates are high2, local recurrence rates after 12 months follow-up can be considerable (21-49%)3–6. Preliminary evidence suggests insufficient ablation margins as a predictor for recurrence after cryoablation3, but current methods to quantify the ablative margin can only be used retrospectively due to their time-consuming nature. Hence, there is a need for a volumetric approach to intraoperatively quantify the ablation margin during the actual procedure. In this work, we present a novel deep learning based algorithm for near real-time monitoring of the ablative margin and perform an initial retrospective validation of its correlation with local outcome.Methods
This retrospective study was approved by the IRB and informed consent was waived. All cryoablation procedures at our institution were performed on a clinical 3-T MRI system (Magnetom Skyra, Siemens). At the beginning of each cryoablation procedure biplane T2-weighted turbo spin echo imaging and diffusion-weighted imaging were performed for anatomical reference and target tumor re-identification. After cryoprobe placement, cryoablation was performed using two 10:3 min freeze-thaw cycles. During ablation, Ice ball progression was continuously monitored using T1-weighted volume interpolated gradient echo (VIBE) imaging (TR/TE=4.8/1.4ms FA=6°; slice thickness = 2.5 mm; no of slices = 26; Tacq 0:46 min) until the end of the last ablation cycle.An ablative margin analysis algorithm was developed consisting of several processing steps (Figure 1). Prior to this work, a 2D U-net7 was trained for ice ball segmentation on 205 intraprocedural T1 VIBE MR scans, achieving a mean dice coefficient on the test set of 0.96±0.02. Similarly, an existing multimodal medical image registration model (VoxelMorph8) was trained for T2w-T1w prostate MR image registration using a dataset of 76 patients.
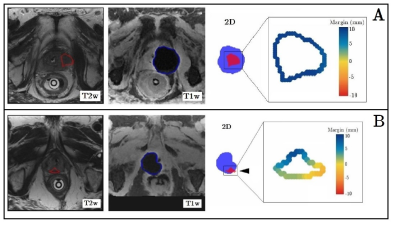
The algorithm was retrospectively applied to data of 27 patients that underwent focal cryoablation for localized primary (n=2) or radiorecurrent (n=25) prostate cancer at our institution and had at least 12 months of follow-up. First, a manual segmentation of the prostate tumor was performed in consensus by two prostate interventionalists on the pre-ablation T2 TSE and DWI images. Second, the registration model registered each intraprocedural T1 VIBE, capturing the growing ice ball extent, with the pre-ablation T2 TSE scan. Third, the segmentation model automatically extracted the iceball on each registered T1w scan. The algorithm then automatically quantified the overlap between tumor and ice ball volume, expressed as a percentage, as well as the minimal ablation margin (MAM), defined as the smallest three-dimensional distance between the iceball and tumor surface. This loop is continuously repeated for each consecutive T1 VIBE scan until the final frozen extent is achieved at the end of the procedure. End-of-ablation tumor coverage and minimal ablation margin were correlated with local outcome. Local tumor progression (LTP) after cryoablation was defined as either a positive targeted biopsy or imaging evidence of local recurrence at the previously treated site during follow-up.
Results
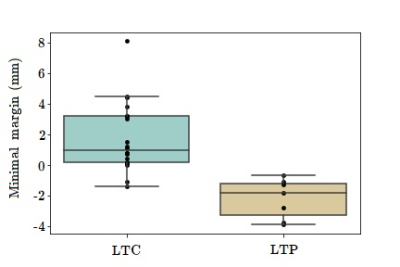
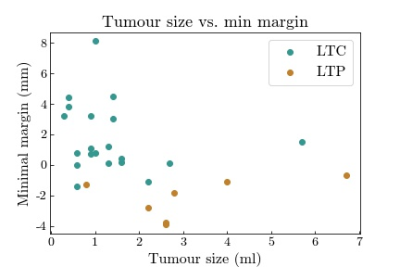
Mean follow-up was 23 months (range: 5-69). LTP was detected in 7/27 (26%) patients. Ablative margin analysis by the algorithm was successful in 24/27 (89%) cases (Figure 2). In two cases registration was inaccurate due to gross motion which was not sufficiently compensated by the registration algorithm. In one case ice ball segmentation failed at an area where the ice ball infiltrated surrounding adipose tissue. After manual correction these cases could be included in the ablative margin analysis. On correlation with local outcome, tumour coverage (97.5%±3.0 vs. 99.8%±0.6, p<0.001) and minimal ablation margin (-2.2±1.2 mm vs. 1.7±2.2 mm, p<0.001) (Figure 3) were significantly smaller for cases with versus without LTP. All cases of LTP had a negative MAM. Tumors with a negative MAM tended to be larger (Figure 4).Discussion
In this work we demonstrate a novel algorithm for automated ablative margin analysis during MR-guided cryoablation procedures. Automatic quantification was successful in 89% of cases and algorithm-derived ablative margin parameters appeared correlated with local outcome. An interesting observation is that two patients did not achieve a tumour coverage of 100% but did not show local recurrence during follow-up. For both cases it was found that a larger ice ball at the site of incomplete coverage could not be obtained due to close proximity to either the urethra or the rectal wall. Therefore, in the case of a slight registration mismatch and an inability of the ice ball to expand further in a given direction, this resulted in an apparent incomplete tumour coverage. Furthermore the algorithm failed in three cases, necessitating further model optimization. Finally, our work is limited by the small sample size, its retrospective nature and potential errors in the segmentation and registration process that require further validation in larger cohorts.Conclusion
We have developed a deep-learning assisted algorithm for near real-time monitoring of the ablative margin during MR-guided prostate focal cryoablation. Initial retrospective validation indicates automatically determined minimal ablative margin and tumour coverage to correlate with local treatment outcome. Prospective use may aid the physician in obtaining adequate ablative margins during prostate cryoablation procedures.Acknowledgements
No acknowledgement found.References
[1] C. Khoo et al., “A systematic review of salvage focal therapies for localised non-metastatic radiorecurrent prostate cancer,” Translational Andrology and Urology, vol. 9, no. 3, 2020, doi: 10.21037/tau.2019.08.21.
[2] J. G. R. Bomers et al., “Focal Salvage MR Imaging–Guided Cryoablation for Localized Prostate Cancer Recurrence after Radiotherapy: 12-Month Follow-up,” Journal of Vascular and Interventional Radiology, vol. 31, no. 1, pp. 35–41, 2020, doi: 10.1016/j.jvir.2019.07.001.
[3] C. G. Overduin, S. F. M. Jenniskens, J. P. M. Sedelaar, J. G. R. Bomers, and J. J. Fütterer, “Percutaneous MR-guided focal cryoablation for recurrent prostate cancer following radiation therapy: retrospective analysis of iceball margins and outcomes,” European Radiology, vol. 27, no. 11, pp. 4828–4836, 2017, doi: 10.1007/s00330-017-4833-9.
[4] J. G. R. Bomers et al., “MR imaging-guided focal cryoablation in patients with recurrent prostate cancer,” Radiology, vol. 268, no. 2, pp. 451–460, 2013, doi: 10.1148/radiol.13121291.
[5] D. A. Woodrum et al., “Magnetic resonance imaging-guided cryoablation of recurrent prostate cancer after radical prostatectomy: Initial single institution experience,” Urology, vol. 82, no. 4, pp. 870–875, 2013, doi: 10.1016/j.urology.2013.06.011.
[6] N. Wake, A. B. Rosenkrantz, D. K. Sodickson, H. Chandarana, and J. S. Wysock, “MRI Guided Procedure Planning and 3D Simulation for Partial Gland Cryoablation of the Prostate : A Pilot Study,” Research Squre, pp. 1–14, 2020, doi: 10.21203/rs.2.23583/v2.
[7] O. Ronneberger, P. Fischer, and T. Brox,“U-net: Convolutional networks forbiomedical image segmentation,” Lect.Notes Comput. Sci. (including Subser.Lect. Notes Artif. Intell. Lect. NotesBioinformatics), vol. 9351, pp. 234–241,2015.
[8] G. Balakrishnan, A. Zhao, M. R.Sabuncu, J. Guttag, and A. V Dalca,“VoxelMorph: A Learning Framework forDeformable Medical Image Registration,”IEEE Trans. Med. Imaging.
Figures