2147
AI-Assisted Analysis of Iceball Coverage on Patient Outcomes Following MR-guided Focal Cryoablation for Prostate Cancer: A Retrospective Study1Department of Radiology, Brigham and Women's Hospital, Boston, MA, United States, 2Boston University School of Medicine, Boston, MA, United States
Synopsis
We conducted a retrospective study of 44 MRI-guided focal cryoablation cases for prostate cancer. Our goal was to evaluate AI-assisted MRI-based assessment of inadvertent cryoinjuries in the surrounding structures as predictors of post-procedural morbidities. Specifically, we estimated the iceball involvement with the external urethral sphincter (EUS) and the neurovascular bundle (NVB) using intraprocedural T2-weighted MRI and correlated with acute postoperative urinary incontinence and erectile dysfunction. The study demonstrated a correlation between incontinence and MRI-estimated iceball involvement with the EUS. However, no correlation was found between erectile dysfunction and the iceball involvement with the NVB.
Purpose
Despite prostate cancer’s prevalence in the American population, treatment options remain limited for those that are diagnosed. The current standards of treatment, including radical prostatectomy and radiation therapy, have been associated with multiple comorbidities[2], and the most aggressive forms of cancer remain resistant to these therapies. Following unsuccessful initial treatment, focal MR-guided cryoablation has been used as a salvage form of treatment for residual lesions[3]. This treatment utilizes MR imaging to precisely identify the boundaries of the tumor and the frozen tissue to achieve the trifecta of continence, potency, and oncologic efficiency[4-6]. This study aims to evaluate the potential of MR-guidance in predicting postoperative complications. Our hypothesis is that potential damage to the external urethral sphincter (EUS) and the neurovascular bundles (NVB) estimated on intraprocedural MRI using an AI-assisted image segmentation can predict acute postoperative operative urinary incontinence and erectile dysfunction.Methods
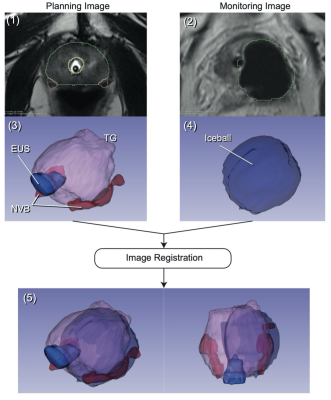
Subjects and Image Data AcquisitionIntraprocedural MR images were acquired for planning and monitoring during MR-guided focal prostate cryoablation treatments (n=44; age: 57-84). A total of 44 planning images and 430 monitoring images were obtained using a T2-weighted TSE sequence (TR/TE = 4800/96ms, matrix = 256⨉179, 3mm slice, FOV = 160⨉160 mm2 for planning; TR/TE = 2430/104ms, matrix = 192⨉134, 3mm slice, 0.6 mm slice gap, FOV = 180⨉157 mm2 for monitoring) (Fig. 1 (1) and (2)).
AI-Assisted Assessment of Ablation Volumes
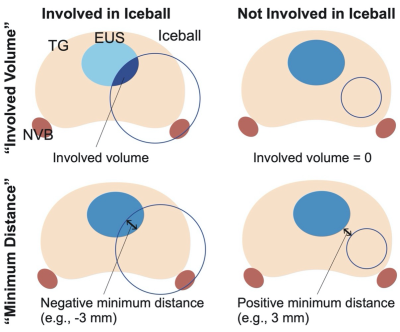
The total gland (TG), EUS, and NVB were segmented automatically on the planning images using a convolutional neural network (CNN) model (Fig. 1). Similarly, the iceball shown as a signal void on the monitoring image was segmented using a separate CNN model. The signal void represents the outer edge of the frozen tissue (the -8 °C isotherm). The models were trained using a dataset manually labeled by a board-certified radiologist prior to the study[7]. The iceball volumes involved with the EUS and NVB (“involved volume”) and the minimum distance from the iceball boundary to the EUS and NVB (“minimum distance”) (Fig. 2) were calculated as metrics to quantify the extent of cryoinjuries in those structures.
Review of Follow-up Data
Chart review was conducted for each case to correlate this data to relevant patient clinical data. Each patient’s electronic health record on EPIC was reviewed for notes from their initial follow-up after their procedure (~14 days post-op). The physician notes and review of systems (ROS) from this visit were used to assess for the presence of postoperative symptoms.
Statistical Analysis
In the analysis of the presence of postoperative symptoms, patients were binarily split into two groups, one group that reported a particular symptom and another group that did not report this symptom. This data was then cross-referenced with the MR-estimated involved volumes in the EUS/NVB and also the MR-estimated minimum distance of the iceball from these structures. This information was then compiled and subjected to Shapiro-Wilk for normality test and Wilcox test to assess for statistical significance.
Results
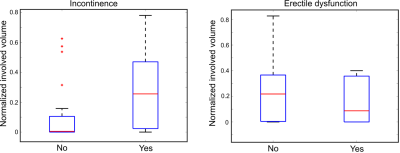
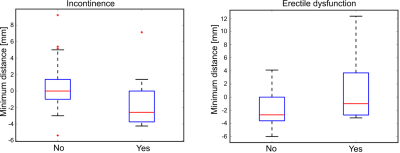
Analysis of the data showed a statistically significant correlation between the involved volume in the EUS and the presence of postoperative urinary incontinence (p=0.028). The analysis of the NVB and erectile dysfunction indicated no statistical difference between the two groups (p=0.45). The normalized involved volume in the EUS for the group that reported symptoms of incontinence was 0.26 [0.04; 0.43] (median [IQR]), while the involved volume for the group that did not report incontinence was 0.01 [0.0; 0.09]. Similar results were obtained when analyzing the minimum distance between the iceball and the EUS/NVB. There was a statistically significant correlation between the minimal distance between the iceball and the EUS and the presence of postoperative urinary incontinence (p=0.025). The minimum distance for the group that reported symptoms of incontinence was -2.5mn [-3.6; -0.5], while the volume for the group that did not report incontinence was 0.0mm [-1.0; 1.4]. The same analysis for the NVB and erectile disfunction indicated no statistical difference between the two groups (p=0.08).Discussion and Conclusion
These results indicate that AI-assisted segmentation of intraprocedural MRI images can be predictive of postoperative symptoms after focal cryoablation of prostate cancer. Further validation and advancement of this process could potentially allow clinicians to more accurately brief patients on expected comorbidities of this procedure based on analysis of intraprocedural imaging. Some potential challenges of this analysis include: reliance of data gathering from secondary reporting of patient symptoms in physician notes upon chart review, which were varied and inconsistent, that the statistical analysis was only based on maximum overlap volume calculations, and the shifting of anatomical structures between the planning and intraprocedural images. Potential future directions include conducting a voxel-wise analysis of the freeze-thaw cycle, which would provide greater detail of the iceball margins and volume. Further, this study assumed uniform temperature distribution throughout the iceball, with any potential areas of overlap equally likely to cause damage. Utilizing temperature-sensitive imaging, demonstrated in our previous study[8], could also allow for more accurate insights into the risks of iceball growth near the EUS/NVB.Acknowledgements
The study was funded in part by the U.S. National Institutes of Health (R01EB020667, P41EB028741). The content of the material is solely the responsibility of the authors and does not necessarily represent the official views of these agencies. Work was also partially funded by the Medical Student Summer Research Program at Boston University School of Medicine.References
[1] Perera M, Krishnananthan N, Lindner U, Lawrentschuk N. An update on focal therapy for prostate cancer. Nat Rev Urol. 2016;13(11):641-653. doi:10.1038/nrurol.2016.177
[2] Wang AZ, Lebastchi AH, O’Connor LP, et al. Making a case “for” focal therapy of the prostate in intermediate risk prostate cancer: current perspective and ongoing trials. World J Urol. 2021;39(3):729-739. doi:10.1007/s00345-020-03525-0
[3] Valerio M, Ahmed HU, Emberton M, et al. The role of focal therapy in the management of localised prostate cancer: a systematic review. Eur Urol. 2014;66(4):732-751. doi:10.1016/j.eururo.2013.05.048
[4] Marien A, Gill I, Ukimura O, Betrouni N, Villers A. Target ablation--image-guided therapy in prostate cancer. Urol Oncol. 2014 Aug;32(6):912-23. doi: 10.1016/j.urolonc.2013.10.014. Epub 2014 Jan 9. PMID: 24411788.
[5] Woodrum DA, Kawashima A, Gorny KR, Mynderse LA. Prostate cancer: state of the art imaging and focal treatment. Clin Radiol. 2017;72(8):665-679. doi:10.1016/j.crad.2017.02.010
[6] Overduin CG, Jenniskens SFM, Sedelaar JPM, Bomers JGR, Fütterer JJ. Percutaneous MR-guided focal cryoablation for recurrent prostate cancer following radiation therapy: retrospective analysis of iceball margins and outcomes. Eur Radiol. 2017;27(11):4828-4836. doi:10.1007/s00330-017-4833-9
[7] Meyer A, Mehrtash A, Rak M, Bashkanov O, Langbein B, Ziaei A, Kibel AS, Tempany CM, Hansen C, Tokuda J. Domain adaptation for segmentation of critical structures for prostate cancer therapy. Sci Rep. 2021 Jun 1;11(1):11480. doi: 10.1038/s41598-021-90294-4. PMID: 34075061; PMCID: PMC8169882.
[8] Tokuda J, Wang Q, Tuncali K, Seethamraju RT, Tempany CM, Schmidt EJ. Temperature-Sensitive Frozen-Tissue Imaging for Cryoablation Monitoring Using STIR-UTE MRI. Invest Radiol. 2020 May;55(5):310-317. doi: 10.1097/RLI.0000000000000642. PMID: 31977600; PMCID: PMC7145748.
Figures