2145
MAGNETIC RESONANCE ACOUSTIC RADIATION FORCE IMAGING (MR-ARFI) FOR THE MONITORING OF MR-GUIDED HIFU THERAPY1Université de Strasbourg, CNRS, ICube, UMR 7357, Strasbourg, France, 2Department of interventional imaging, Hôpitaux Universitaires de Strasbourg, Strasbourg, France
Synopsis
Tissue biomechanical properties are highly promising biomarkers for HIFU ablation monitoring. A quantitative, MR-acoustic radiation force imaging (ARFI)-based method is proposed for measuring both tissue elasticity and viscosity, together with PRFS-derived temperature. This method is based on the identification of the MR-ARFI focal spot pattern. The method was first validated in a phantom with known properties. The method was then evaluated during HIFU heating in a gelatin phantom. Both elastic modulus and viscosity were found to decrease as temperature increased. These results highlight the ability of this method to provide new, quantitative biomarkers of tissue thermal damage in real time.
Introduction
High Intensity Focused Ultrasound (HIFU) therapies are non-ionizing and non-invasive well-established procedures for the targeted destruction of tumours. Monitoring such treatments is fundamental to ensure their safety and efficiency. HIFU therapies are conventionally monitored by MR thermometry. In addition to temperature, the mechanical properties of tissues can be considered as permanent biomarkers of thermal damage1,2. In 2018, Vappou et al1 presented an MR-ARFI-based method for real-time, non-invasive assessment of tissue elasticity changes during HIFU ablations. In this work, substantial refinements of this method are proposed in order to provide both elasticity and viscosity measurements in real time. Both the quantitative nature of the proposed method and its ability to monitor an HIFU ablation in a gel phantom are investigated.Methods
Based on the MR-ARFI method3, the HIFU transducer performing the therapy is also used to induce a micrometric displacement in the focal zone, resulting in the propagation of a transient shear wave of velocity cs (Fig.1). This wave is encoded on the MRI phase signal through the use of motion sensitizing gradients (MSG) synchronized to the acoustic radiation force push through the so-called toffset parameter (Fig.1). Identification of the experimental MR-ARFI phase profile to a theoretical one, makes it possible to determine cs and therefore the shear modulus μ of the medium1 (Fig.2). The theoretical MR-ARFI profile is modelled following:$$ \Delta\phi_{th}(x,y)=\gamma\int_{0}^{T_{MSG}}G(t)\delta(x,y,t)dt $$
where γ is the gyromagnetic ratio of hydrogen, G is the MSG amplitude, TMSG its duration and δ is the displacement generated within the focal zone. To model tissue viscoelasticity, δ is assumed to follow a 1st order exponential law of time constant τ (Fig.2) representing the mechanical relaxation time of the tissue, considered as locally homogeneous.
In this study, we propose to further refine the MR-ARFI real-time monitoring in order to estimate both μ and τ, through the modelling of the geometric attenuation of the shear wave and the real MSG time profile. All experiments were performed on a 1.5T MRI system (Magnetom Aera, Siemens, DE). Main acquisition parameters of the segmented EPI GRE sequence were: TR/TE 500/28ms, FOV 256mm×256mm, matrix 128×128, ST 5mm, EPI factor 37, coronal orientation, MSG 30mT/m-10ms-slice encoding direction. Two images are acquired per toffset, with opposite MSG polarity in order to double the phase to noise ratio in MR-ARFI (phase difference image) and retrieve temperature through PRFS thermometry (average phase)4.
The HIFU system is a 128-element spherical transducer with a nominal focal length of 6cm. The ARFI push duration was set equal to TMSG/2 = 5ms.
Firstly, the validation of the method is performed in a homogeneous commercial calibrated phantom (CIRS, USA). The value of reference for shear elastic modulus is 5.9kPa ±5%. Regarding the mechanical relaxation time τ, the value of reference is determined following the approach published by Kaye et al5 and Dadakova et al6, which consists in fitting the phase signal at the focal spot within a range of values of toffset. With ARFI shots set at 160W acoustic power, the toffset is varied from -5 to 1ms, every 1ms, these measures being repeated 3 times.
Secondly, HIFU heating (40W acoustic power, duty cycle 74% ie 370ms /TR 500ms) interleaved with ARFI shots (5ms, 200W acoustic power) (Fig.4) is performed in a tissue-mimicking phantom (porcine gelatine+ condensed milk) The proton resonance frequency shift (PRFS) method corrected for B0 drift is used to measure temperature changes from the same dataset4. Monitoring was performed in real time using a single toffset = -2ms.
Results
In the homogenous calibrated phantom, the value of reference determined for τ is 3.5ms ±4%. The estimated μ and τ were stable over the explored range of toffset and the 3 repetitions (Fig.3), and were found equal to 6.4kPa ±9% and 2.5ms ±24%, respectively, averaged over all 3 repetitions and individual toffset (Fig.3).During the HIFU ablation in the gelatin phantom, the MR-ARFI spot size was found to decrease versus time, suggesting that the gel softens as a result of heating (Fig.5). Indeed, both μ and τ decreased from 6.5kPa to 2.5kPa and from 6ms to 2ms, respectively (Fig.5). Both viscoelastic parameters were estimated every 4s with the current acquisition parameters.
Discussion
Excellent agreement was found between the μ and τ estimated with the MR-ARFI approach and their respective values of reference in the commercial phantom. The proposed method allows estimating simultaneously the mechanical relaxation time, the shear modulus and relative temperature, at no additional cost in terms of acquisition time compared to the original method.The monitoring experiment allowed us to identify changes in tissue viscoelasticity during HIFU heating. It appeared that the gel started to melt locally after 100s of ablation, corresponding to a temperature increase of 20°C above room temperature, preventing the shear waves from propagating properly afterwards.
Conclusion
Thanks to its ability to perform quantitative viscoelasticity measurements non-invasively and in real time around the focal spot, the presented quantitative MR-ARFI-based method is particularly interesting for monitoring HIFU therapies. Further experiments in ex vivo tissues are needed.Acknowledgements
This work has benefitted from funding of the FUI (Fonds Unique Interministériel, BPI France) for the UFOGUIDE project, and the ANR (Agence Nationale de la Recherche) French national program “Investissements d’Avenir” for the LABEX-CAMI (ANR-11-LABX-004) and the IHU Strasbourg (Institute of Image Guided Surgery, ANR-10-IAHU-02).
References
[1] J. Vappou, P. Bour, F. Marquet, V. Ozenne, and B. Quesson, ‘MR-ARFI-based method for the quantitative measurement of tissue elasticity: application for monitoring HIFU therapy’, Phys. Med. Biol., vol. 63, no. 9, p. 095018, 04 2018
[2] T. Wu, J. P. Felmlee, J. F. Greenleaf, S. J. Riederer, and R. L. Ehman, ‘Assessment of thermal tissue ablation with MR elastography’, Magn. Reson. Med., vol. 45, no. 1, pp. 80–87, 2001
[3] N. McDannold and S. E. Maier, ‘Magnetic resonance acoustic radiation force imaging’, Med. Phys., vol. 35, no. 8, pp. 3748–3758, Aug. 2008
[4] N. Corbin et al., ‘Interventional MR elastography for MRI-guided percutaneous procedures: Interventional MR Elastography’, Magn. Reson. Med., vol. 75, no. 3, pp. 1110–1118, Mar. 2016
[5] E. A. Kaye and K. B. Pauly, ‘Adapting MRI Acoustic Radiation Force Imaging For In Vivo Human Brain Focused Ultrasound Applications’, Magn. Reson. Med. Off. J. Soc. Magn. Reson. Med. Soc. Magn. Reson. Med., vol. 69, no. 3, pp. 724–733, Mar. 2013
[6] T. Dadakova, A. J. Krafft, A. C. Özen, and M. Bock, ‘Optimization of acoustic radiation force imaging: Influence of timing parameters on sensitivity’, Magn. Reson. Med., vol. 79, no. 2, pp. 981–986, 2018
Figures
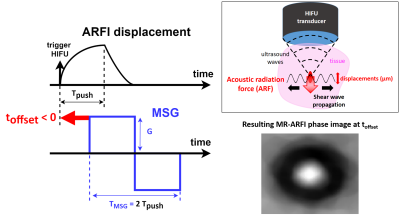
Fig.1 General concepts of MR-ARFI. Left: timings and synchronisation between ARFI and motion sensitizing gradients (MSG). Top right: acoustic radiation force (ARF) causing a shear wave to propagate through the tissue, away from the focal spot. Bottom right: circular shear wave pattern in the MR-ARFI phase image acquired perpendicular to the ultrasound beam axis.
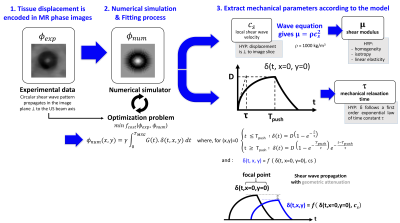
Fig.2 Identification process of the viscoelastic properties using the proposed MR-ARFI-based method: experimental ARFI profiles are fitted by theoretical ARFI profiles using a viscoelastic shear wave propagation model. The best solution of the optimization problems yields both shear elastic modulus µ and viscoelastic relaxation time τ.
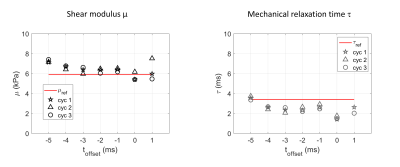
Fig.3 Validation experiment in a homogenous commercial phantom. Shear modulus μ (left) and mechanical relaxation time τ (right) obtained with the MR-ARFI model for 7 different toffset, with 3 repetitions (cyc 1 to 3 in legend). Horizontal red lines are drawn at the reference values, i.e. the phantom datasheet for µ, and reference τ determined from the phase at the focal spot for a range of toffset5,6. Good agreement and reproducibility are found for both µ and τ in this range of toffset.
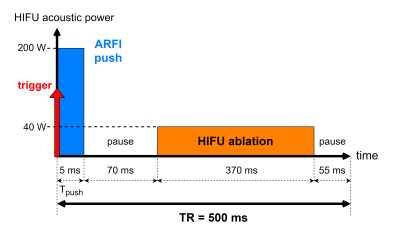
Fig.4 Chronogram of the focused ultrasound sequence: interleaved ARFI shot (blue) and HIFU ablation (orange) for the monitoring with MR-ARFI of an HIFU ablation in the gelatine phantom.
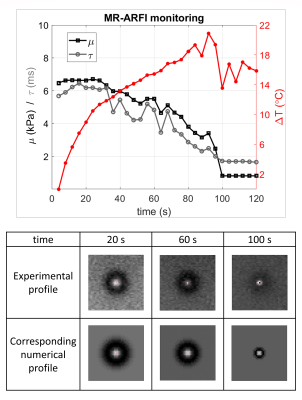
Fig.5 MR-ARFI monitoring in the gelatine phantom during HIFU ablation. Top: time evolution of the shear modulus μ (black), the mechanical relaxation time τ (grey), and the temperature (red) estimated with MR-ARFI during HIFU ablation in the gelatine phantom. Bottom: 3 selected experimental MR-ARFI profiles during HIFU, and their corresponding identified numerical profiles. The ARFI spot diameter decreases as temperature increases, corresponding to decreasing viscoelastic properties µ and τ.