2144
Estimating acoustic intensity in viscoelastic media using MR acoustic radiation force imaging and multifrequency MR elastography1Electrical Engineering, Stanford University, Stanford, CA, United States, 2Radiology, Stanford University, Stanford, CA, United States
Synopsis
In addition to focal spot localization for non-ablative transcranial ultrasound therapies, MR acoustic radiation force imaging (MR-ARFI) has the potential to estimate the acoustic intensity delivered to the target. However, variability in tissue stiffness and viscosity precludes accurate estimates of acoustic intensity from MR-ARFI displacements alone. We demonstrate that multifrequency MRE can be used to characterize a medium’s viscoelastic response to focused ultrasound, improving estimates of acoustic intensity and enabling safer, more effective treatments.
Introduction
Skull heterogeneity presents a significant challenge towards consistently safe and effective transcranial focused ultrasound (FUS) therapies. The same applied power resulted in vastly different outcomes (up to 4×) across patients that underwent FUS thalamotomy1. While magnetic resonance (MR) thermometry provides invaluable thermal dose and targeting information for ablative FUS therapies, there remains a need for improved in situ acoustic intensity estimates for non-ablative FUS therapies, such as neuromodulation.MR acoustic radiation force imaging (MR-ARFI) was developed for focal spot localization without an associated temperature rise, and it could potentially provide the means for acoustic intensity estimation2, as the acoustic radiation force is proportional to the acoustic intensity3. However, MR-ARFI alone cannot reliably determine acoustic intensity. The influence of acoustic transmission through the skull on MR-ARFI displacements cannot be separated from the effects of tissue stiffness and viscosity, meaning the same acoustic radiation force could result in considerably different encoded displacements depending on the tissue’s viscoelastic properties, which vary across patients, brain regions, and disease states4–7. Using viscoelastic phantoms, we demonstrate this confound and propose a method for estimating acoustic intensity in viscoelastic media using MR-ARFI displacements alongside mechanical properties obtained from multifrequency MR elastography (MRE).
Methods
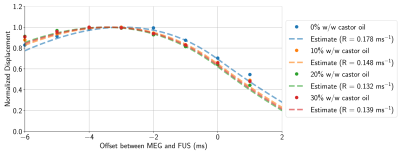
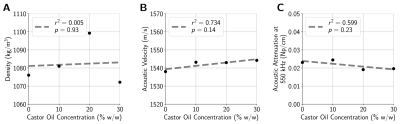
Following a protocol similar to Farrer et al.8, tissue-mimicking phantoms were fabricated by dissolving 100-bloom gelatin (6% w/w) in a suspension of deionized water, castor oil, and evaporated milk (50% w/w). Phantom viscosity was modulated by varying the proportion of castor oil from 0% w/w to 40% w/w. Each phantom’s density, acoustic velocity, and attenuation at 550 kHz were measured using a through-transmission method.MR-ARFI was performed with a 2DFT spin-echo sequence (FOV = 20 cm × 20 cm, acquisition matrix = 256×128, slice thickness = 0.7 mm, TE = 39 ms, TR = 500 ms) at 3T (Signa Excite, GE Healthcare, Milwaukee, WI). Displacements induced by a 550 kHz, 16 ms ultrasound pulse (ExAblate 2100, Insightec Ltd., Haifa, Israel) were encoded using repeated bipolar MEGs9. Using four applied power levels ranging from 50 W to 200 W, spatial-peak pulse-average acoustic intensities were measured with a hydrophone (Precision Acoustics, Dorset, UK) in free water, and de-rated using the acoustic attenuation measured from each phantom to obtain in situ acoustic intensity estimates. To characterize the time-varying displacement response of each phantom, additional MR-ARF images were acquired by varying the delay between the start of FUS and the start of the second MEG lobe from -6 ms to 1 ms. Displacement responses were assumed to follow an inverse exponential curve3, and the relaxation rate of the response was estimated by minimizing the mean squared error between measured and simulated displacements.
MRE was performed by inducing shear waves within each phantom using a passive driver, and encoding resultant displacements with sinusoidal MEGs and a single-shot, spin-echo, echo-planar imaging sequence4 (FOV = 22 cm × 22 cm, acquisition matrix = 128×128, slice thickness = 3.5 mm, acceleration factor = 2×, TE = 58.6 ms, TR = 2000 ms). Maps of the dynamic shear modulus were reconstructed using a 3D direct inversion algorithm at four actuation frequencies: 40 Hz, 60 Hz, 70 Hz, and 80 Hz. Mean storage and loss moduli were measured across a 1 cm3 region centered at the focal spot, and these moduli were used to estimate frequency-independent Voigt model elasticity and viscosity parameters.
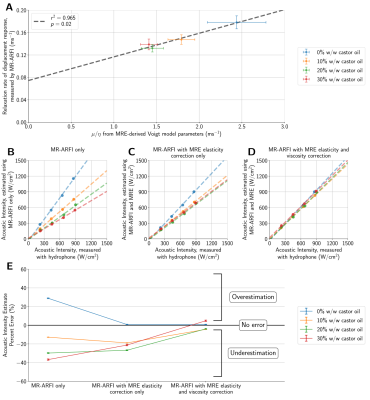
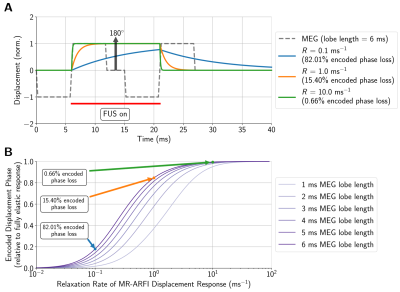
Linear weightings based on estimated Voigt model elasticity parameters were applied to MR-ARFI displacements to produce initial acoustic intensity estimates. Next, relaxation rates of the time-varying displacement response were estimated from Voigt model viscosity parameters and used to compute secondary correction factors using the loss in encoded displacement relative to the encoded displacement phase for a purely elastic material (Figure 1).
Results
The applied acoustic radiation force is proportional to the acoustic properties of the medium3, however, the variation in acoustic properties across phantoms was minimal (Figure 2). Phantoms fabricated with more castor oil had higher Voigt model elasticity and viscosity parameters as well as a lower relaxation rate parameter (Figure 3). Similarly, relaxation rates estimated from ARF-induced displacement responses also decreased with increasing castor oil concentration (Figure 4) and were strongly correlated with Voigt model relaxation rate parameters (Figure 5A, r2 = 0.965).When estimating acoustic intensity with solely MR-ARFI, the average absolute error across all phantoms was 27.1% (Figure 5). The mean absolute error was reduced to 17.3% when using elasticity parameters estimated from MRE, with acoustic intensities being underestimated in more viscous phantoms. Finally, mean absolute error was reduced to 3.8% when accounting for the effects of viscosity on the relaxation rate of the response.
Discussion
Our results demonstrate the potential for MR-ARFI displacements to estimate the acoustic intensity at the focal spot when used in combination with information about the tissue’s viscoelastic properties from MRE. Furthermore, improved characterization of the time-varying displacement response with multifrequency MRE could be useful for optimizing MR-ARFI pulse sequences to achieve higher displacement signal-to-noise ratio for the same ultrasound exposure. As the applied acoustic radiation force has an inertial component related to the focal spot size10, future studies will investigate the impact of aperture configuration and phase aberration due to the skull on acoustic intensity estimates.Acknowledgements
This work was supported by NIH T32 CA009695, T32 EB009653, and NSF DGE 1656518. We would like to thank Patricia Sheu Lan for her assistance acquiring MR elastography images, as well as Richard L. Ehman and Kevin Glaser from the Mayo Clinic for providing the MR elastography sequence and passive driver.References
1. Vyas U, Ghanouni P, Halpern CH, Elias J, Pauly KB. Predicting variation in subject thermal response during transcranial magnetic resonance guided focused ultrasound surgery: Comparison in seventeen subject datasets. Med Phys. 2016;43(9):5170-5180. doi:10.1118/1.4955436
2. Gerstenmayer M, Rémi M, Fellah B, Denis LB, Larrat B. Magnetic resonance acoustic radiation force imaging for in vivo estimation of ultrasonic transmission factor through rat skulls. In: The 16th International Symposium on Therapeutic Ultrasound. ; 2016:374-375.
3. Nightingale KR, Palmeri ML, Nightingale RW, Trahey GE. On the feasibility of remote palpation using acoustic radiation force. J Acoust Soc Am. 2001;110(1):625-634. doi:10.1121/1.1378344
4. Pepin KM, McGee KP, Arani A, et al. MR elastography analysis of glioma stiffness and IDH1-mutation status. Am J Neuroradiol. 2018;39(1):31-36. doi:10.3174/ajnr.A5415
5. Arani A, Murphy MC, Glaser KJ, et al. Measuring the effects of aging and sex on regional brain stiffness with MR elastography in healthy older adults. Neuroimage. 2015;111:59-64. doi:10.1016/J.NEUROIMAGE.2015.02.016
6. Sack I, Beierbach B, Wuerfel J, et al. The impact of aging and gender on brain viscoelasticity. Neuroimage. 2009;46(3):652-657. doi:10.1016/J.NEUROIMAGE.2009.02.040
7. Murphy MC, Huston J, Jack CR, et al. Decreased brain stiffness in Alzheimer’s disease determined by magnetic resonance elastography. J Magn Reson Imaging. 2011;34(3):494-498. doi:10.1002/JMRI.22707
8. Farrer AI, Odéen H, de Bever J, et al. Characterization and evaluation of tissue-mimicking gelatin phantoms for use with MRgFUS. J Ther Ultrasound. 2015;3(1):9. doi:10.1186/s40349-015-0030-y
9. Bitton RR, Kaye E, Dirbas FM, Daniel BL, Pauly KB. Toward MR-guided high intensity focused ultrasound for presurgical localization: Focused ultrasound lesions in cadaveric breast tissue. J Magn Reson Imaging. 2012;35(5):1089-1097. doi:10.1002/jmri.23529
10. Viola F, Walker WF. Radiation force imaging of viscoelastic properties with reduced artifacts. IEEE Trans Ultrason Ferroelectr Freq Control. 2003;50(6):736-742. doi:10.1109/TUFFC.2003.1209564
Figures
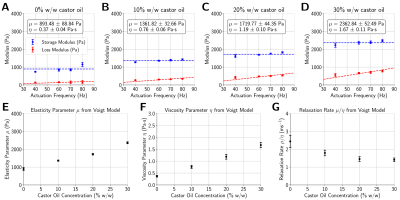
(A-D) Using reconstructed maps of the dynamic shear modulus from multifrequency MRE, storage and loss moduli were measured at the targeted location in each phantom, and Voigt model parameters were estimated with linear least-squares regression. (E, F) Voigt model elasticity (μ) and viscosity (η) parameters increased with castor oil concentration, while (G) the relaxation rate (μ/η) parameter decreased.