2143
Acquiring and Reconstructing UTE-Dixon Fat and Water Images for Generation of Synthetic CT in Thorax on an MR-Linac1Radiotherapy and Imaging, Institute of Cancer Research, London, United Kingdom
Synopsis
Generation of synthetic CT (synCT) for adaptive MR-guided radiotherapy is particularly challenging in the thorax due to the impact of respiratory motion, short-T2* tissues in lung, and a complex tissue-density structure. To address these challenges and provide data suitable for generating thoracic synCTs, we developed a UTE-Dixon sequence with a golden-angle stack-of-stars trajectory. We have demonstrated successful online acquisition and offline reconstruction of UTE, fat, water and B0 images as well as retrospective respiratory gating. Work is ongoing to optimize processing of respiratory-resolution of UTE and Dixon images that will form the input to synCT in thorax on an MR-Linac.
Purpose
Synthetic CT (synCT) is a key component of adaptive MR-guided radiotherapy. Thoracic synCT, however, is particularly challenging1 due to the impact of respiratory motion, short-T2* tissues in lung, and a complex tissue-density structure. To address these respective challenges will ideally require respiratory-resolved images that provide suitable contrast in lung (such as UTE images2) and are multiparametric such that they may be used as an input in a deep-learning-based approach3 to overcome challenging heterogeneity of the thorax. Here, we present our work towards thoracic 4D-synCT for MR-guided radiotherapy based on UTE imaging and motion-resolved fat, water, and B0 maps.Methods
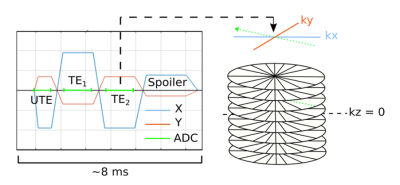
Data Acquisition: A three-point Dixon UTE sequence with non-selective excitation (Figure 1) was run on a 1.5-T MR-Linac system (Unity, Elekta AB, Stockholm) with two 4-channel phased array coils for a healthy volunteer. A 3D stack-of-stars trajectory was employed with parameters: UTE/TE1/TE2 0.14/1.8/3.5 ms, TR 7.9 ms, 60 image slices, resolution 1.5 x 1.5 x 5 mm, field-of-view 50 x 50 x 30 cm, 664 spokes, acquisition time 415 s, bandwidth/pix 865 Hz. A kz-oversampling factor of 1.72 was employed to mitigate wrap-around artifacts resulting from non-selective excitation.Trajectory Correction: K-space trajectories were corrected for gradient delays by convolving the gradient waveform with the gradient impulse response function4 in MATLAB (MathWorks, Natick, MA) as previously described.5 UTE and bipolar-echo images were reconstructed using the corrected k-space trajectories with an adjoint non-uniform fast Fourier transform.6
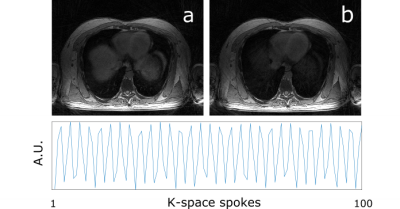
Respiratory Resolving: The first non-UTE k-space data was used for self-gating via Principal Component Analysis (PCA) on the Fast Fourier Transform (FFT) along kz of the central point of each spoke. Data were sorted by respiratory signal in five respiratory bins with 50% data overlap between adjacent respiratory bins and respiratory-resolved images were reconstructed with XD-GRASP7 in MATLAB.
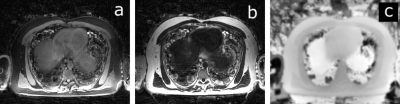
Fat-Water Reconstruction: UTE-Dixon fat and water images and B0 maps were reconstructed using the Dixon-RAVE algorithm8 adapted to work with center-out UTE raw data.
Results
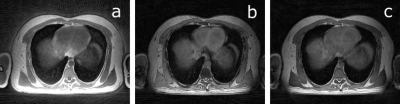
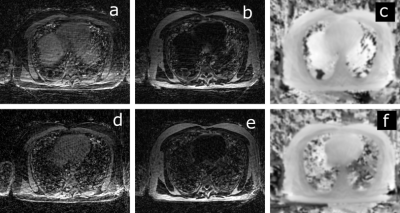
Fully sampled UTE, and bipolar-echo images are shown in Figure 2, for a slice at the level of the diaphragm. Raw data from the first non-UTE echo was used for detection of the respiratory signal shown in Figure 3 alongside inspiration and expiration images reconstructed with the XD-GRASP technique.Figure 4 illustrates motion-averaged water, fat, and B0 maps generated from the adapted Dixon-RAVE reconstruction method for the full acquisition. In lung, spurious signal is found, potentially associated with errors in the estimated initial guess for the B0 map. Figure 5 shows motion resolved images, where the Dixon-RAVE technique was applied to inhalation and exhalation respiratory bins. Images are noticeably noisier within the lungs; however, respiratory motion can be seen.
Discussion
We successfully acquired and reconstructed in vivo UTE-Dixon water, fat, and B0 maps from a sequence designed for online generation of thoracic synCT on an MR-Linac system. SynCT is necessary for adaptive MR-guided radiotherapy since replanning requires simulations in tissue density models, normally provided by CT, to calculate the distribution of radiation dose. Generation of synCT onboard a hybrid MR-Linac allows on-the-fly replanning with a greater degree of certainty than co-registration to previously acquired CT scans.9While synCT is a solved problem for sites that are relatively static and of relatively homogenous tissue density such as brain10-11 and pelvis,12-13 thoracic anatomies are more challenging. First, not only it is necessary to avoid respiratory motion artifacts, but it is also desirable to characterize the motion in free-breathing to inform MR-Linac techniques that use gating or trailing14 of the treatment beam. Second, the short T2* of lung also presents a challenge since it is dosimetrically relevant to distinguish regions of emphysema from healthy parenchymal tissue. Our solution is to employ UTE imaging that can achieve signal in such rapidly decaying tissue components. Third, the complex distribution of thoracic tissue densities means that simple approaches, such as bulk-density assignment for synCT generation are not feasible. Instead, a voxelwise approach that requires multiparametric images is likely to be optimal, such as deep-learning generative adversarial networks, where the network can potentially also account for the confounding effect of artifacts and noise.
In future work, we will further augment our 4D-UTE-Dixon reconstruction by reducing artifacts by jointly reconstructing several respiratory states using an XD-Dixon-Rave8 approach or employing motion compensation.15 This will allow us to build a clinically feasible method for 4D synCT in thorax on the MR-Linac.
Acknowledgements
No acknowledgement found.References
1. Freedman JN, Bainbridge HE, Nill S, et al. Synthetic 4D-CT of the thorax for treatment plan adaptation on MR-guided radiotherapy systems. Phys. Med. Biol. 2019;64.
2. Su KH, Friel HT, Kuo JW, et al. UTE-mDixon-based thorax synthetic CT generation. Med. Phys. 2019;46:3520–3531.
3. Spadea MF, Maspero M, Zaffino P, Seco J. Deep learning based synthetic-CT generation in radiotherapy and PET: A review. Med. Phys. 2021;1–30.
4. Vannesjo SJ, Haeberlin M, Kasper L, et al. Gradient system characterization by impulse response measurements with a dynamic field camera. Magn. Reson. Med. 2013;69:583–593.
5. Goodburn R, Bruijnen T, Bano W, Oelfke U, Wetscherek A (2021) Measuring the Gradient Impulse Response Function (GIRF) for UTE Imaging on an MR-Linac. In: (Proceedings) 29th Annual Meeting of the Int. So. Mag. Reson. Med.
6. Fessler JA, Sutton BP. Nonuniform fast Fourier transforms using min-max interpolation. IEEE Trans. Signal Process. 2003;51:560–574.
7. Feng L, Axel L, Chandarana H, Block KT, Sodickson DK, Otazo R. XD‐GRASP: Golden‐angle radial MRI with reconstruction of extra motion‐state dimensions using compressed sensing. Magn. Reson. Med. 2015;75:775–788.
8. Benkert T, Feng L, Sodickson DK, Chandarana H, Block KT. Free-breathing volumetric fat/water separation by combining radial sampling, compressed sensing, and parallel imaging. Magn. Reson. Med. 2016;78:565–576.
9. Edmund JM, Nyholm T. A review of substitute CT generation for MRI-only radiation therapy. Radiat. Oncol. 2017;12:1–15.
10. Lee J, Carass A, Jog A, Zhao C, Prince JL. Multi-atlas-based CT synthesis from conventional MRI with patch-based refinement for MRI-based radiotherapy planning. In: Proc SPIE Int Soc Opt Eng. ; 2017. p. 10133.
11. Han X. MR-based synthetic CT generation using a deep convolutional neural network method: Med. Phys. 2017;44:1408–1419.
12. Chen S, Qin A, Zhou D, Yan D. Technical Note: U-net-generated synthetic CT images for magnetic resonance imaging-only prostate intensity-modulated radiation therapy treatment planning. Med. Phys. 2018;45:5659–5665.
13. Maspero M, Savenije MHF, Dinkla AM, et al. Dose evaluation of fast synthetic-CT generation using a generative adversarial network for general pelvis MR-only radiotherapy. Phys. Med. Biol. 2018;63:0–11.
14. Fast M, van de Schoot A, van de Lindt T, Carbaat C, van der Heide U, Sonke J-J. Tumor Trailing for Liver SBRT on the MR-Linac. Int. J. Radiat. Oncol. Biol. Phys. 2019;103:468–478.
15. Rank CM, Heußer T, Buzan MTA, et al. 4D respiratory motion-compensated image reconstruction of free-breathing radial MR data with very high undersampling. Magn. Reson. Med. 2016;77:1170–1183.
Figures