2142
Developing and testing robotic MRI/CT fusion biopsy using a novel home-made interventional phantom1Radiology, Royal Marsden Hospital, London, United Kingdom, 2Institute of Cancer Research, London, United Kingdom, 3MRI Unit, Royal Marsden Hospital, London, United Kingdom
Synopsis
MRI/CT fusion may enable MRI-guided biopsy without the requirement for dedicated interventional MRI facilities. Assessment and training in MRI/CT-fusion biopsy requires phantoms containing targets that can be seen on MRI but not on CT and can be biopsied. These requirements are not met using commercially available phantoms. We produced a phantom containing targets that can be appreciated on MRI but not CT, which is reproducible, can be biopsied and assessed for core adequacy. We successfully biopsied targets (1-3cm diameter) using a commercially available interventional robot equipped with work-in-progress MRI/CT-fusion software, including targets with steep out-of-plane angulations, within clinically reasonable timeframes.
INTRODUCTION
The soft-tissue contrast and functional information provided by MRI1 may enable targeted biopsies of heterogeneous tumours, and could enhance our understanding of MRI signal including imaging biomarker validation. However, biopsy is conventionally performed under ultrasound- or CT-guidance due to lower cost, availability, and ease of use2. MRI/CT-fusion provides a potential solution but remains largely undeveloped in Interventional Radiology. A CT-guided interventional robot with multiplanar planning, stereotactic targeting and MRI/CT-fusion is available but has not been used clinically. Guidelines for new interventional procedures3 recommend preclinical research before clinical use, but commercially-available phantoms are costly, cannot be biopsied, and have components which can be seen on both MRI and CT, limiting investigation of MRI/CT-fusion.The aim of this study is to develop a reproducible MRI/CT-fusion biopsy phantom containing targets that can be seen on MRI but not CT that can be biopsied and assessed for core adequacy. We then use the phantom to assess feasibility of a new MRI/CT-fusion biopsy robot.
MATERIALS AND METHODS
Gelatine, agar, and mixed gelatine/agar were investigated for tumour (target) and background materials4, in silicone trays (100ml cube per sample) (Figure 1). Biopsies (16G) were taken, and core quality assessed visually.Trays were imaged using a 1.5T MRI scanner (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany) in a bath of doped water (770mg.L-1 CuSO4, 2000mg.L-1 NaCl). T1 relaxation times were estimated using inversion-prepared turbo spin-echo (TSE) images (inversion times 35-3000ms); T2 relaxation times were estimated using a multi-contrast spin-echo sequence (echo times 10-320ms) described previously5. Trays were imaged twice on Day 1 (2 hours apart) for repeatability, and Day 8 for medium-term stability. Trays were stored in a refrigerator and placed in the scanner room 24 hours before imaging.
T1 and T2 were estimated using in-house software (Adept, ICR). Curves were fitted pixel-by-pixel and median values in a square region-of-interest (750mm2) calculated for each sample. Hounsfield Units (HU) were assessed using CT scans (SOMATOM Definition Edge, Siemens) of the same trays on Days 1 and 8. Repeatability of T1, T2 and HU was assessed using Bland-Altman analysis6.
Biopsy phantom construction
Biopsy phantoms (Figure 2) were constructed using Perspex boxes (25cm x 25cm x 15cm) filled with background (non-target) material, with four sets of three spherical ‘tumour’ targets (diameters 1cm, 2cm, 3cm) at 10cm depth. A stamp was used to create wells in the background material for reproducible target positioning. Targets were coloured for assessment of biopsy cores. A square aperture (13.5cm x 13.5cm) in the lid mandated in-plane biopsy (targets 1–3); single out-of-plane approaches (targets 4–6 and 7–9), and double out-of-plane (targets 10–12).
Two phantoms (A/B) were constructed (one week apart) for reproducibility assessment. Phantoms were imaged using a 3D T2-weighted TSE (axial, slice thickness 2.5mm, TE=140ms, TR=1500ms, in-plane reconstructed pixel size=1.3mm x 1.3mm). External markers were used to align the phantom. Reproducibility of biopsy phantom images was assessed qualitatively using image fusion (Horos, horosproject.org) and quantitatively using Dice similarity coefficient (DSC, Matlab 2021a, Mathworks, Natick, MA).
Biopsy
A commercially-available interventional robot (MAXIO™; Perfint Healthcare, Chennai, India) (described previously7) was used, with work-in-progress MRI/CT-fusion software. Biopsy of all targets was attempted twice (2 days apart) by a Consultant Interventional Radiologist (4 years' experience, including 1 month in robotic intervention) (Figure 3). Radiation dose, duration, and number of scans were recorded. Planned-tip-to-actual-tip deviation was recorded for negative biopsies.
RESULTS
HU, T1, T2HU, T1, and T2 demonstrated strong dependence on gelatine and agar concentrations (Figure 4b-d). Repeatability of HU, T1, and T2 was good, with upper and lower 95% LoA 14.1% and -3.8% (HU), 5.5% and -2.8% (T1), and 1.7% and -1.7% (T2) (Figure 4e-g).
1:10-gelatine background material, and 1:10-gelatine/6:100-agar target material provided contrast between T2 values, matched HU, and good quality biopsy cores, and were used for subsequent biopsy phantom construction.
Phantom
Figure 4 illustrates the excellent MRI contrast between targets and background, and absence of CT contrast. Size and position of targets was highly reproducible (Figure 4l) with DSC between Phantoms A/B comparable to repositioning the same phantom.
Biopsy
23/24 biopsies (96%) yielded target material (Figure 5). The single negative biopsy was the 1cm in-plane target at first attempt, which had 0mm planned-tip-to-actual-tip deviation, consistent with planning error.
DISCUSSION
We developed a low-cost, reproducible phantom for MRI/CT-fusion, which can be biopsied and used to assess core adequacy. The main advantage of our phantom over commercially-available products is the visibility of targets on MRI but not CT, allowing evaluation of MRI/CT-fusion biopsy before clinical studies.Biopsies using a commercially-available robot were accurate and consistently achieved cores from 1cm targets including >20°angulations out of two planes (XYZ). These results compare favourably with conventional freehand biopsy where diagnostic yield for lesions <1cm is limited8–11. Procedural duration was clinically acceptable.
Phantoms allow assessment of intra- and inter-operator error, without inter-patient variation. Limitations include absence of motion and lack of resemblance to patient anatomy. Further work will test technique reproducibility with other operators before translation to patients.
CONCLUSION
We successfully developed a low-cost, reproducible biopsy phantom, which was used to test a robotic technique for MRI/CT-fusion biopsy. Biopsies were accurate, and achievable in clinically acceptable timescales.Acknowledgements
Funding was obtained from the Royal of College of Radiologists, Pump Priming Grant.
This study represents independent research supported by the National Institute for Health Research (NIHR) Biomedical Research Centre and the Clinical Research Facilities at The Royal Marsden NHS Trust and the Institute of Cancer Research, London. The views expressed here are those of the author(s) and not necessarily those of the National Health Service, the National Institute for Health Research or the Department of Health.
References
1. D G Mitchell, D L Burk Jr, S Vinitski, M. D. R. The biophysical basis of tissue contrast in extracranial MR imaging. Am. J. Roentgenol. 149, 831–7 (1987).
2. Veltri, A., Bargellini, I., Giorgi, L., Almeida, P. A. M. S. & Akhan, O. CIRSE Guidelines on Percutaneous Needle Biopsy (PNB). Cardiovasc. Intervent. Radiol. 40, 1501–1513 (2017).
3. Hirst, A. et al. No Surgical Innovation Without Evaluation: Evolution and Further Development of the IDEAL Framework and Recommendations. Ann. Surg. 269, 211–220 (2019).
4. Zettinig, O. et al. Multimodal image-guided prostate fusion biopsy based on automatic deformable registration. Int. J. Comput. Assist. Radiol. Surg. 10, 1997–2007 (2015).
5. Stupic, K. F. et al. A standard system phantom for magnetic resonance imaging. Magn. Reson. Med. 86, 1194–1211 (2021).
6. Bland, J. M. & Altman, D. G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1, 307–310 (1986).
7. Koethe, Y., Xu, S., Velusamy, G., Wood, B. J. & Venkatesan, A. M. Accuracy and efficacy of percutaneous biopsy and ablation using robotic assistance under computed tomography guidance: A phantom study. Eur. Radiol. 24, 723–730 (2014).
8. Stattaus, J. et al. CT-guided biopsy of small liver lesions: Visibility, artifacts, and corresponding diagnostic accuracy. Cardiovasc. Intervent. Radiol. 30, 928–935 (2007).
9. Chang, Y. Y., Chen, C. K., Yeh, Y. C. & Wu, M. H. Diagnostic feasibility and safety of CT-guided core biopsy for lung nodules less than or equal to 8 mm: A single-institution experience. Eur. Radiol. 28, 796–806 (2018).
10. Huang, M. De et al. Accuracy and complications of CT-guided pulmonary core biopsy in small nodules: A single-center experience. Cancer Imaging 19, 1–10 (2019).
11. Tian, P. et al. CT-guided transthoracic core needle biopsy for small pulmonary lesions: Diagnostic performance and adequacy for molecular testing. J. Thorac. Dis. 9, 333–343 (2017).
Figures
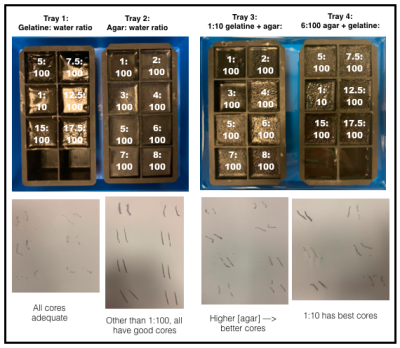
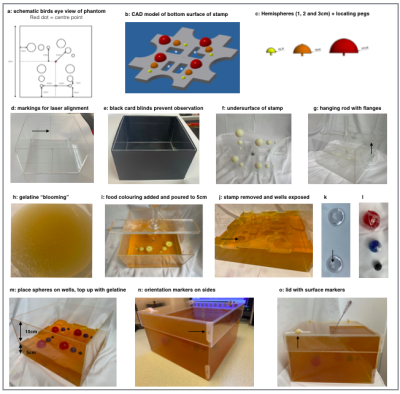
Figure 2.
a-c: Main plate (grey) + coloured hemispheres. Defects (asterisks) enable removal, reducing interfacial tension.
d-e: Box.
f: Plate.
g: Flanges (arrow) keep stamp 5cm from base.
h: 1:10 gelatine:water heated to 50°C in water bath.
i: Stamp in place.
j: Wells for targets.
k: Two hemispherical domes form each sphere. 2mm hole in apex accommodates 18G needle (arrow). 6:100 agar mixed with an equal volume of 1:10 gelatine.
l: Red, blue, and black food colouring added to 3, 2 and 1cm targets respectively.
m-o: phantom.
Steps h–o were carried out in a domestic kitchen in less than a day.

Figure 3.
a: Coronal/birds-eye T2-weighted TSE with numbered targets.
b: Phantom on CT table next to robot.
c: Phantom aligned and fixed with adhesive putty.
d: CT/MRI-fusion using fiducial markers, and needle path planning using robot workstation.
e: CT (left), fused MR/CT (middle) and MRI (right).
f: Robot and inserted co-axial.
g: Radiologist performing biopsy.
h: Biopsy core with target (black) and non-target surround (very pale yellow). Total and cancer core lengths measured by ruler.
i: Robot workstation showing planned (left), actual (right) and fused needle paths (middle).
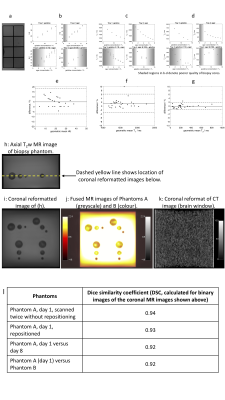
Figure 4.
a: Coronal MR image (tray 2).
b: HU, c: T1 and d: T2 vs. gelatine/agar concentrations. Bland-Altman plots showing differences between two measurements vs. means, e: HU, f: T1, g: T2. Solid lines = mean difference. Dashed lines = 95% LoA. HU and T1 showed small but significant differences between repeated measurements (4.7%, 1.3%); the slight T1 increase may be due to warming during scanning.
Biopsy phantom:
h: axial MRI, i: coronal reformat, j: fused MRI of Phantoms A and B, k: CT, l: DSC.
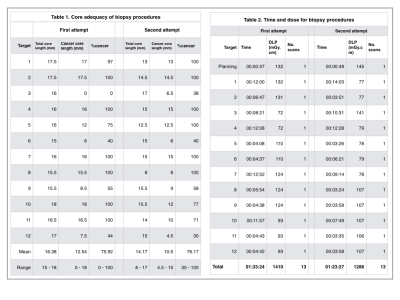
Figure 5.
Results of the robotic biopsy study.