2141
Establishing a passive motor fMRI protocol for intraoperative 3T MRI: Preliminary results
Gilbert Hangel1, Jonathan Wais2, Matthias Tomschik2, Gudrun Mayr-Geisl2, Philip Pruckner3, Pedro Cardoso4, Christian Dorfer2, Gregor Kasprian3, and Karl Rössler2
1Department of Neurosurgery & High Field MR Centre, Medical University of Vienna, Wien, Austria, 2Department of Neurosurgery, Medical University of Vienna, Vienna, Austria, 3Division of Neuroradiology and Musculoskeletal Radiology, Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria, 4Medical University of Vienna, Vienna, Austria
1Department of Neurosurgery & High Field MR Centre, Medical University of Vienna, Wien, Austria, 2Department of Neurosurgery, Medical University of Vienna, Vienna, Austria, 3Division of Neuroradiology and Musculoskeletal Radiology, Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria, 4Medical University of Vienna, Vienna, Austria
Synopsis
Using a 3T ioMRI system with an 8-channel intraoperative coil, we developed passive sensorimotor intraoperative fMRI protocol under anaesthesia at our site. We successfully applied it to passive fMRI of hand and foot in an exploratory cohort of four patients, confirming correspondence of activation to preoperative fMRI scans. This is the first reported implementation of intraoperative 3T passive fMRI.
Purpose
Intraoperative fMRI offers high utility for the localisation of brain function in cases of acute surgery or low patient compliance (due to mental status or age), can account for brain shift, and potentially predict postoperative functionality. Previous research has demonstrated imaging of the sensorimotor cortex using passive hand or finger motion using 1.5T intraoperative MRI (ioMRI) and found still significant BOLD activation in small patient cohorts1,2. Benefits from using 3T instead of 1.5T systems are relevant contrast improvements3 that could increase reproducibility or reduce scan time under anaesthesia. Due to the limited availability of 3T ioMRI so far, passive fMRI was only demonstrated with equal activation to active movement in awake volunteers4.Using a recently installed 3T ioMRI setup with an 8-channel intraoperative coil, we explored the feasibility and methodological possibilities of a passive sensorimotor intraoperative fMRI (pifMRI) protocol under anaesthesia and surgical limitations at our site.
Methods
For this study, we used a 3T Skyra (Siemens Healthineers, Erlangen, Germany) system with XQ gradients (45 mT/m gradient strength and 200 mT/m/s slew rate). In our dual-room setup, it is adjacent to a neurosurgery suite and equipped with a dockable patient transfer table. In addition to a 64-channel head coil for preoperative scans, we used a combined 8-channel coil and head holder (NORAS, Hochberg, Germany) for intraoperative scans. For task synchronisation, we used an optical stimulus system (NordicNeuroLab, Bergen, Norway).First, we determined optimal possible EPI sequence parameters for the 8-channel coil, especially acceleration for TR reduction, with healthy volunteer scans. Less coil elements than regular contemporary head coil arrays mean less sensitivity and fewer possibilities for parallel signal acquisition. Starting with a TR of 4 s and coverage of the cerebrum, we tested parallel imaging and multiband excitation possibilities in order to reduce volume acquisition time and obtain a close to optimal TE.
We then applied the resulting pifMRI setup to intraoperative scans of four patients (36f, 23f, 56m, 42f) undergoing tumour resection in proximity to the primary sensorimotor cortex. Clued by the stimulus system, an operator moved the subject’s corresponding wrist or ankle (extension, flexion) once during each active volume, identical to awake active motor tasks (Figure 1). Morphological T1 and T2-weighted sequences were acquired. We measured this protocol before and after gross resection. As reference, all patients received a presurgical fMRI protocol in the week before surgery with active and passive motion of the respective appendages. These scans featured a TR of 1 s, 2.5 mm isotropic voxel size filtered to 3 mm, 51 slices, a TE of 30 ms, and a block design with 300 volumes (30 alternating volumes on/off), 5 min scan time, and finger tapping or ankle motion. The acquired pifMRI data was correlated and validated using electrical motor cortex stimulation and presurgical fMRI (activation maps were all calculated using Philips Dynasuite during/after surgical planning).
Results
In the current state of optimization after the first volunteer scans, we arrived at an EPI scan with the following features: Multiband factor 2 and slice acceleration factor 2 with 24 reference lines for a TR of 2 s, 3 mm isotropic resolution filtered to 3.3 mm, at least 24 slices, a TE of 30 ms, a block design with 135 volumes (15 alternating volumes on/off), and 4:30 min scan time.The application to the four intraoperative patient scans was realised successfully without complications during pifMRI. An example of intraoperative contrast and image quality both before and after resection is given in Figure 2. The results showed activation consistent to preoperative fMRI and motor cortex stimulation in the primary sensorimotor areas in all cases, before and after resection. Figure 3 gives an overview of the correspondence of activation derived from all scans, while Figure 4 shows the validation of passive stimulus during preoperative awake fMRI. We further achieved processing of pre-resection pifMRI into surgical navigation maps and transfer to neuronavigation within the timeframe of surgery, as demonstrated in Figure 5.
Discussion
We achieved both goals of finding a feasible 3T fMRI protocol for intraoperative use and applying it to mapping of the sensorimotor cortices in a small cohort of patients. To our knowledge, this is the first reported implementation of 3T pifMRI. We could accelerate EPI with an 8-channel intraoperative coil enough to achieve a TR of 2 s, allowing an acquisition in 4:30 min that can realistically be integrated into an ioMRI workflow. Distortion artefacts were observed and need to be addressed in the future. Passive fMRI in general allows sensorimotor localisation in patients who for any reason lack the ability to perform fMRI motor tasks. Compared to previous studies at 1.5T1,2, we achieved shorter TRs and therefore more volumes per time and potentially increased contrast. 3T pifMRI appears to be a promising addition to ioMRIOur current limitations are the preliminary status of the protocol, a small sample size, and head placement within the coil frame restricted by surgical needs. Thorough evaluation of the detected activation under consideration of image distortions caused by surgical procedures, larger cohorts and sophisticated statistical analysis will be necessary to define the performance gain compared to 1.5T and clinical utility satisfactorily.
Acknowledgements
No acknowledgement found.References
1Gasser et al., Neurosurgery 2005, doi: 10.1227/01.neu.0000163488.91335.c5
2Yamamoto et al., NeuroImage: Clinical 2019, doi: 10.1016/j.nicl.2019.101923
3Triantafyllou et al., PLOS ONE 2011, doi: 10.1371/journal.pone.0024519
4Blatow et al., J Magn Reson Imaging, doi: 2011, 10.1002/jmri.22629
Figures
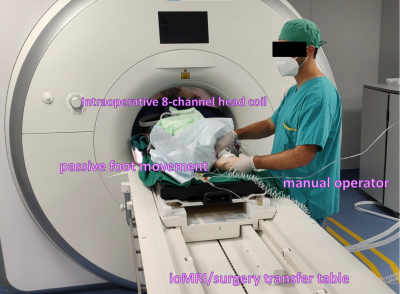
Figure
1: Setup for intraoperative passive motor fMRI for the left foot in a patient
post-resection. The manual operator receives clues for movement timing by the
fMRI system monitor.
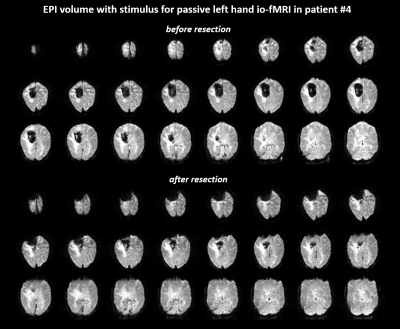
Figure
2: Comparison of EPI BOLD contrast for
intraoperative passive motor fMRI before and after resection in patient #4 (41
years, female, palsy of the left lower extremity, postsurgical histology showed
metastasis concordant to a non-small-cell lung cancer). The shown volume 20/135
was acquired during passive motion.
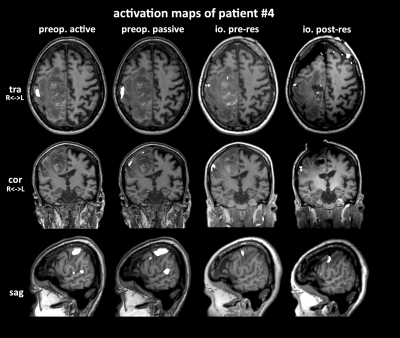
Figure
3: Comparison of activation maps of active and passive motor fMRI before
surgery, and pifMRI before and after resection. pifMRI activation is smaller,
but well aligned with awake fMRI. Post-resection activation is noticeably
affected by brain shift.
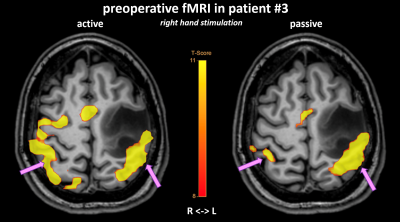
Figure
4: Validation of passive fMRI of the right hand in a preoperative scan using
the 64-channel head coil in patient #3 (56 years, male, high-grade glioma).
Passive hand movement in an awake state produces less activation than active
hand movement, but still localises the same cortical regions.
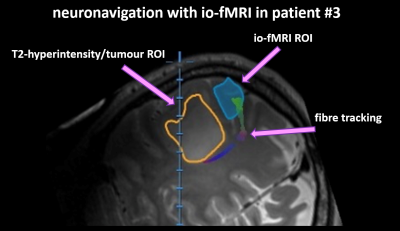
Figure 5: Integration of intraoperative passive
fMRI into neuronavigation for the purpose of surgical planning (patient #3 with
a left-hemispheric mass-lesion in proximity to the sensorimotor cortex). MRI
shows T2-hyperintensity caused by the peritumoral edema (orange) as well as the
ROI defined by io-fMRI (blue) with the corresponding fibre-tracks.
DOI: https://doi.org/10.58530/2022/2141