2138
Intraoperative MRI-derived 3D ablation margins and correlation with local outcome after MRI-guided cryoablation of small renal tumors1Medical Imaging, Radboudumc, Nijmegen, Netherlands, 2Urology, Radboudumc, Nijmegen, Netherlands, 3Fraunhofer Institute for Digital Medicine, Bremen, Germany
Synopsis
MRI-guided cryoablation of small renal tumors relies on achieving sufficient ice ball coverage throughout the entire lesion for effective treatment. However, a volumetric approach to assess treatment margins intraoperatively is currently lacking. This work retrospectively derived three-dimensional ablation margins after cryoablation in 39 kidney tumors using co-registration of intraoperatively acquired pre- and post-ablation MR images. Minimal ablation margins were significantly smaller for cases with versus without local tumor progression. Radiological complete coverage (ablation margin >0 mm) was associated with local control. MRI-derived 3D ablation margins may present a useful intraprocedural tool to assess treatment success.
Introduction
Percutaneous cryoablation is an accepted treatment alternative in patients with small renal tumors1. MRI-guidance allows several advantages over standard computer tomography (CT)-guidance, including the capability of high soft-tissue contrast without contrast medium, multiplanar imaging and near real-time monitoring of volumetric ice ball progression2. The capability to visualize the entire ice ball volume enables image-based assessment of volumetric tumor coverage but a validated intraoperative approach is currently lacking. In practice, most interventionalists aim for a treatment margin of 5 mm to minimize the risk for local recurrent disease3. Recent studies however have suggested renal tumors may be effectively treated at smaller treatment margins of 1-1.5 mm4-5, but were limited to two-dimensional measurements or CT-based delineations. Therefore, the purpose of this work was to retrospectively assess three-dimensional (3D) minimal ablation margins after MRI-guided percutaneous cryoablation of small renal tumors using intraoperative MR-MR image co-registration and determine its correlation with local treatment success.Methods
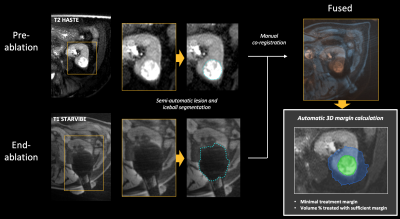
Between May 2014 and January 2021, 34 patients (mean age: 69y) underwent percutaneous MRI-guided cryoablation at our institution for 39 small renal tumors (size: 1.6-5.1 cm) and were at least 6 months after treatment. All procedures were performed under general anesthesia on a 3-T clinical MR system (Magnetom Skyra, Siemens). Intraprocedural pre-ablation imaging consisted of bi-plane T2-weighted half fourier spin echo (HASTE) imaging in apnea when possible. Needle insertion was performed under ultrasound guidance at a safe zone within the MRI-suite or in case of poor visibility on ultrasound under direct MR imaging guidance. After confirmation of probe placement with MRI, cryoablation was performed in two 10:3 minute freeze-thaw cycles. Continuous monitoring of ice ball progression was performed using biplanar T2 HASTE or T1-weighted stack of stars volume interpolated gradient echo (STARVIBE) acquisitions, where the final acquisition was performed at the end of ablation when possible in apnea. After the procedures, tumor and ice-ball volumes were semi-automatically segmented on the intraprocedural pre- and corresponding end-of-ablation MR images using Software Assistant for Interventional Radiology (SAFIR) research software (Fraunhofer, Germany). Using the same software, pre- and post-ablation MR images were aligned using manual landmark-based rigid co-registration, after which volumetric ablation margins were automatically quantified (Figure 1). The minimal ablation margin (MAM) was defined as the smallest 3D distance between the tumor and ice-ball surface. Local tumor progression (LTP) after cryoablation was assessed on follow-up imaging.Results
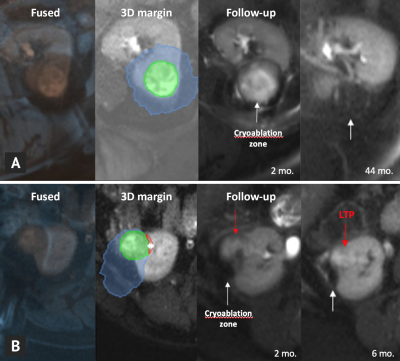
Median follow-up was 20 months (range: 1-58). Local control after cryoablation was achieved in 33 tumors (85%) while LTP occurred in 6 (15%). Retrospective analysis of the 3D ablation margin using intraoperative pre- and post-ablation MR image co-registration was feasible in all patients (100%) (Figure 2). Minimal ablation margin was significantly smaller for cases with (-6.9±3.5 mm) vs. without LTP (2.3±1.9 mm) (P<.001) (Figure 3). No LTP was observed in patients with a MAM ≥0 mm. Cases with LTP had significantly larger tumor diameters (4.1±0.5 vs. 2.9±0.8 cm, P<.001). All negative treatment margins occurred in patients with tumor size >3 cm (Figure 4).Discussion
Two recent studies have indicated a MAM of 1-1.5 mm to be sufficient for effective renal cryoablation4-5. Although a safety margin remains preferable, our preliminary clinical data indicates a MAM ≥0 mm to be predictive of local tumor control. In part, these findings may be due to the ability to assess the treatment margin in 3D, which may in some areas be smaller than the recommended 5 mm without leading to local tumor recurrence. In addition, cryoablation is known to cause direct thermal damage as well as delayed cytotoxic effects, mainly due to thrombosis causing local ischemia6. These effects may not yet be captured on the intraprocedural MRI visualizing the total ice extent, and possibly contribute to a smaller intraprocedurally determined treatment margin to be sufficient for complete ablation. Contrary to previous studies that have used contrast-enhanced CT or 1-day post-treatment MRI, the present method relies on non-contrast intraoperative MRI and therefore shows potential for intraprocedural use. Main limitations of the current work are its limited sample size, single observer and potential inaccuracies arising from the annotation and co-registration process that require further validation in larger cohorts.Conclusion
Volumetric assessment of the periablational treatment margin using intraoperative MR-MR image fusion was feasible and can be useful as an intraprocedural tool to evaluate local treatment success of MR-guided cryoablation for small renal tumors. Ablations with incomplete radiological coverage (ablation margin <0 mm) were associated with local tumor progression.Acknowledgements
No acknowledgement found.References
1. Ljungberg et al. Eur Urol. 2019;75(5):799-810.
2. Mogami et al. Int J Clin Oncol. 2007;12(2):79-84
3. Ahmed et al. Radiology 2014;273(1):241–260
4. Ge et al. J Vasc Interv Radiol. 2016;27(3):403-9.
5. Fraisse et al. BJU Int. 2019;123(4):632-8.
6. Erinjeri et al. J Vasc Interv Radiol. 2010;21(8 Suppl):S187-91
Figures