2135
Shutter Speed Model based Quantitative DCE-MRI for Therapeutic Response Assessment of Pancreatic Ductal Adenocarcinoma: A Pilot Study1Medicine, University of Alabama at Birmingham, Birmingham, AL, United States, 2The University of Texas at Austin, Austin, TX, United States, 3Vanderbilt University, Nashville, TN, United States, 4University of Texas at Austin, Austin, TX, United States, 5University of Alabama at Birmingham, Birmingham, AL, United States
Synopsis
Shutter Speed Model (SSM) based Ktrans, ve, kep, and τi in the pancreatic tumors before initiating chemotherapyt were 0.081±0.036 mm-1, 0.258±0.070, 0.320±0.152 mm-1, and 0.247±0.197 μs, respectively, without statistical difference between the responding and non-responding groups. The Ktrans in the tumors favorably responding to chemotherapy increased 72±14%, significantly larger than in the non-responding tumors (2±9%, p=0.0001). The ve in the responding tumors also significantly increased compared to the non-responding group (64±25% vs 3±9%, p=0.0036). However, no statistical difference was found between the responding and non-responding groups in either kep (6±11% vs 11±16%, p=0.6204) or τi (3±47% vs 147±280%, p=0.3408).
Purpose
The Shutter Speed Model (SSM), a pharmacokinetic (PK) model used in dynamic contrast-enhanced resonance imaging (DCE-MRI), takes into account the fact that the exchange rate of water molecules between cells and extracellular space is limited (1). Therefore, SSM may allow more accurate PK-parameter assessment. As demonstrated in our previous study (2), pancreatic ductal adenocarcinoma (PDAC) is typically hypo-perfused, but effective chemotherapy can increase tumor perfusion. This pilot study tested whether SSM-based PK parameters could detect favorable PDAC response to chemotherapy early.Methods
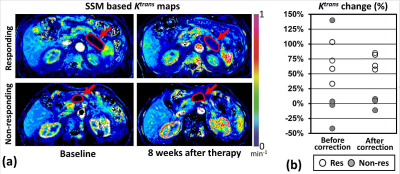
We recruited eight patients having advanced PDAC (one female, seven males, median age = 66 years). Each subject had two DCE-MRI exams before and at 8±1 weeks after starting chemotherapy (either FOLFIRINOX or Abraxane/Gemcitabine) in a 3T scanner (either SIEMENS Prisma or GE Signa). All subjects were imaged together with portable perfusion phantoms developed in our lab for scanner-dependent error correction (3). Board-certified radiologists demarcated the tumor boundary, and the tumor response was determined based on RECIST. Four SSM-based PK maps (Ktrans: Volume transfer constant, ve: Fractional extravascular extracellular volume, kep: Flux rate constant, τi: Mean intracellular water life) were created after phantom-based error correction. The arterial input function (AIF) was estimated using the modified population-based AIF method developed in our lab (4). The change of each PK parameter in the tumors that favorably responded to chemotherapy was compared with that of non-responding tumors with one-way ANOVA.Results
The tumor size (longest axis) was 38±17 mm (mean±SD) in the baseline images, and four subjects responded to chemotherapy. Baseline tumor SSM-based Ktrans, ve, kep, and τi were 0.081±0.036 mm-1, 0.258±0.070, 0.320±0.152 mm-1, and 0.247±0.197 μs, respectively, without statistical difference between the responding and non-responding groups. The Ktrans in the responding group increased 72±14%, significantly larger than in the non-responding group (2±9%, p=0.0001) (Fig 1). The ve in the responding group also significantly increased compared to the non-responding group (64±25% vs 3±9%, p=0.0036). However, no statistical difference was found between the responding and non-responding groups in either kep (6±11% vs 11±16%, p=0.6204) or τi (3±47% vs 147±280%, p=0.3408).Discussion
This is the first report that SSM-based Ktrans and ve can detect PDAC favorably responding to chemotherapy. Effective chemotherapy reduces the cell density, increasing ve, and as a result, the Ktrans is also increased. Since both the Ktrans and ve would increase simultaneously, the kep (kep=Ktrans/ve) would minimally vary. Of interest, the τi variation during the chemotherapy was markedly different between the two groups, but the τi in PDAC suffered from the low signal-to-noise ratio. These findings will need to be confirmed in larger prospective clinical studies.Acknowledgements
No AcknowledgementsReferences
1. Li X, Huang W, Yankeelov TE, Tudorica A, Rooney WD, Springer CS, Jr. Shutter-speed analysis of contrast reagent bolus-tracking data: Preliminary observations in benign and malignant breast disease. Magn Reson Med. 2005;53(3):724-9. Epub 2005/02/22. doi: 10.1002/mrm.20405. PubMed PMID: 15723402.
2. Kim H, Morgan DE, Schexnailder P, Navari RM, Williams GR, Bart Rose J, Li Y, Paluri R. Accurate Therapeutic Response Assessment of Pancreatic Ductal Adenocarcinoma Using Quantitative Dynamic Contrast-Enhanced Magnetic Resonance Imaging With a Point-of-Care Perfusion Phantom: A Pilot Study. Invest Radiol. 2019;54(1):16-22. Epub 2018/08/24. doi: 10.1097/RLI.0000000000000505. PubMed PMID: 30138218.
3. Kim H, Mousa M, Schexnailder P, Hergenrother R, Bolding M, Ntsikoussalabongui B, Thomas V, Morgan DE. Portable perfusion phantom for quantitative DCE-MRI of the abdomen. Med Phys. 2017;44(10):5198-209. Epub 2017/07/12. doi: 10.1002/mp.12466. PubMed PMID: 28692137; PMCID: PMC5646228.
4. Kim H. Modification of population based arterial input function to incorporate individual variation. Magn Reson Imaging. 2018;45:66-71. Epub 2017/09/30. doi: 10.1016/j.mri.2017.09.010. PubMed PMID: 28958876; PMCID: PMC5709187.
Figures