2134
Staging Lymphedema with Dual Agent Relaxivity Contrast Magnetic Resonance Imaging (DARC-MRL)1Stanford University, Stanford, CA, United States
Synopsis
This retrospective cross-sectional study assessed different methods for staging lymphedema on Dual Agent Relaxivity Contrast MR Lymphangiography (DARC-MRL). Images from 20 patients (40 limbs) with clinically diagnosed and staged lower extremity lymphedema who had undergone DARC-MRL were assessed for dermal backflow, lymphatic vessel enhancement, and subcutaneous edema, in six regions of the lower limbs. These assessments were translated into a dermal backflow score and an edema score of our own design, as well as a recently published MRL Stage (Soga et al. 2021). All three scores demonstrated strong to excellent correlation with International Society of Lymphology (ISL) clinical stages.
Introduction
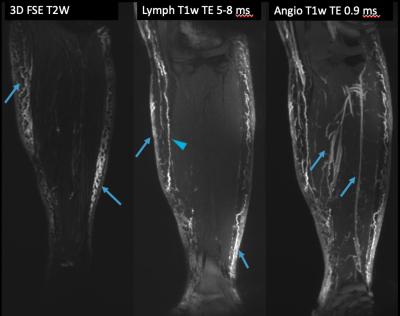
The lymphatic system transports interstitial fluid, lymphocytes, cellular debris, and proteins, out of peripheral tissues. Lymphedema occurs when this transport function fails and can be primary (congenital) or secondary (traumatic, iatrogenic) in origin. It is estimated that over 250 million people worldwide suffer from lymphedema.1 Failure to diagnose and manage lymphedema can result in major adverse clinical outcomes such as progression of disease, loss of mobility, and hospitalizations for cellulitis.2MR lymphangiography (MRL) allows evaluation of peripheral lymphatics.3–5 In single agent MRL, a gadolinium based contrast agent is injected intracutaneously into the distal extremity. The contrast agent is then collected by the lymphatic system and tracked as it is transported centrally. However, the clinically available gadolinium containing contrast agents are suboptimal for lymphatic imaging as their size (~1nm, ~1kDa) allows for diffusion into the blood vasculature, creating lack of differentiation between lymphatic and venous vessels.3,6 Dual agent relaxation contrast (DARC) MRL overcomes this vascular contamination by administering ferumoxytol intravenously.7 Ferumoxytol is an iron oxide agent with an r2/r2* relaxivity approximately an order of magnitude greater than gadolinium. When combined with relatively long echo time 3D SPGR T1-weighted imaging (TE ~5-10 ms), the R2* relaxivity of ferumoxytol suppresses all signal from the vasculature system, allowing gadolinium contrast within the lymphatic system to be imaged in isolation.7-9
Dermal backflow and decreased enhancement of proximal lymphatic vessels are common findings of lymphedema seen in indocyanine green lymphography, lymphoscintigraphy, and conventional pedal lymphangiography.10–13 While a single agent MRL staging system for lymphedema has recently been described that correlates with clinical stage14, no such work has been performed for DARC-MRL. In our project we evaluated several imaging based lymphedema staging systems for DARC-MRL, and correlated these to International Society of Lymphology (ISL) Stage.15
Methods
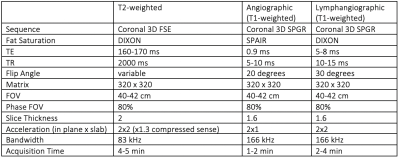
This was an IRB approved retrospective study involving 20 patients (15 female, 5 male) with clinically diagnosed lymphedema (14 with unilateral lower extremity lymphedema, 6 with bilateral lower extremity lymphedema) who underwent DARC-MRL between 2019-2021 as part of their routine clinical care. DARC-MRL technique (relevant sequences in Table 1, example in Figure 1) was based on prior literature7, and was performed on a 3T magnet (GE 750w or Signa Architect). Following pre-contrast imaging, 510 mg ferumoxytol was infused intravenously over 15 minutes, and 4-5 doses of 1-2 mL dilute gadolinium contrast (5:4:1 gadobenate dimeglumine:saline:1% lidocaine) were injected intracutaneously in each foot. Injection sites were massaged, and the lower extremities mobilized, prior to post-contrast imaging.DARC-MRL images were retrospectively evaluated in all 40 lower limbs of the patients. ISL stage was obtained from the clinical record. One radiologist with 5 years of experience retrospectively analyzed all images. Each lower limb was divided into six areas: foot, distal leg, distal thigh, proximal thigh, and groin. The presence or absence of dermal backflow, subcutaneous edema, and visualized lymphatics were identified at every region. Every region with dermal backflow was given 1 point, and the sum of points for each limb was calculated for a dermal backflow score. A similar calculation was performed to calculate an edema score. The MRL stage(1-7) was assessed based on prior literature14, briefly, dermal backflow and visualized lymphatics were assessed in the foot, leg, and thigh with different combinations of findings leading to seven stages. The dermal backflow score, edema score, and MRL stage were compared with clinical assessed lymphedema severity (ISL stage) using a two-tailed Fisher’s exact test. Pearson correlation test was used to assess the correlation between dermal backflow score, edema score, and MRL stage, with ISL stage. Pearson’s rho values of <0.25, 0.25–0.59, 0.50-0.75, and 0.75-1.0 were considered to indicate low, moderate, strong, and excellent correlations, respectively. Significance was set at p<0.05.
Results/Discussion
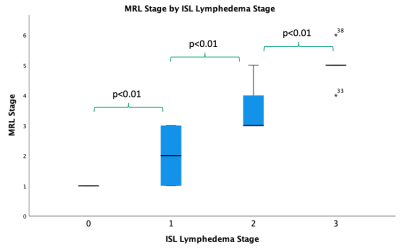
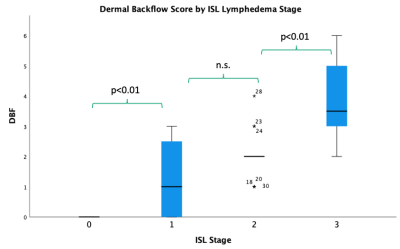
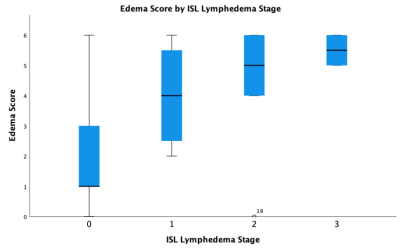
This study revealed the MRL stage when applied to DARC-MRL excellently and significantly correlates with ISL lymphedema stage (Figure 2). Additionally, dermal backflow score alone resulted in an excellent and significant correlation to ISL stage (Figure 3). The authors find the dermal backflow score easier to assess than MRL stage. However, MRL stage was significantly different when compared across each adjacent ISL lymphedema stage, suggesting this method to be a more robust system for staging lymphedema in a manner that correlates with clinical stage. The edema score had a strong and significant correlation with ISL stage (Figure 4). However, no significant difference was observed between edema scores and adjacent ISL lymphedema stages. Limb edema is thought to be more variable depending on time of day and recent therapies such as compression or manual lymph drainage, therefore this finding may be expected. The edema score may be a useful biomarker for symptomatic edema, it could be a useful tool for short term response to therapies, and unlike the dermal backflow score and MRL stage does not require the administration of contrast.Conclusion
MRL stage, dermal backflow score, and edema score, computed from DARC-MRL, correlate with ISL lymphedema clinical stage. Future studies to examine the association of these imaging scores and treatment outcome or prognosis are needed. MRL continues to prove an essential tool that is underutilized for diagnosing, staging, and treatment planning for patients with lymphedema.Acknowledgements
No acknowledgement found.References
1. Rockson SG, Rivera KK. Estimating the Population Burden of Lymphedema. Annals of the New York Academy of Sciences. 2008;1131(1):147-154. doi:10.1196/annals.1413.014
2. Moffatt CJ, Franks PJ, Doherty DC, et al. Lymphoedema: an underestimated health problem. QJM. 2003;96(10):731-738. doi:10.1093/qjmed/hcg126
3. Lohrmann C, Foeldi E, Bartholomä JP, Langer M. Interstitial MR lymphangiography—A diagnostic imaging method for the evaluation of patients with clinically advanced stages of lymphedema. Acta Tropica. 2007;104(1):8-15. doi:10.1016/j.actatropica.2007.07.001
4. Lohrmann C, Foeldi E, Speck O, Langer M. High-Resolution MR Lymphangiography in Patients with Primary and Secondary Lymphedema. American Journal of Roentgenology. 2006;187(2):556-561. doi:10.2214/AJR.05.1750
5. Ruehm SG, Schroeder T, Debatin JF. Interstitial MR Lymphography with Gadoterate Meglumine: Initial Experience in Humans. Radiology. 2001;220(3):816-821. doi:10.1148/radiol.2203010090
6. Notohamiprodjo M, Baumeister RGH, Jakobs TF, et al. MR-lymphangiography at 3.0T—a feasibility study. Eur Radiol. 2009;19(11):2771-2778. doi:10.1007/s00330-009-1461-z
7. Maki JH, Neligan PC, Briller N, Mitsumori LM, Wilson GJ. Dark Blood Magnetic Resonance Lymphangiography Using Dual-Agent Relaxivity Contrast (DARC-MRL): A Novel Method Combining Gadolinium and Iron Contrast Agents. Current Problems in Diagnostic Radiology. 2016;45(3):174-179. doi:10.1067/j.cpradiol.2015.08.003
8. Neligan PC, Kung TA, Maki JH. MR lymphangiography in the treatment of lymphedema. Journal of Surgical Oncology. 2017;115(1):18-22. doi:10.1002/jso.24337
9. Ripley B, Wilson GJ, Lalwani N, Briller N, Neligan PC, Maki JH. Initial Clinical Experience with Dual-Agent Relaxation Contrast for Isolated Lymphatic Channel Mapping. Radiology. 2018;286(2):705-714. doi:10.1148/radiol.2017170241
10. Pappalardo M, Cheng MH. Lymphoscintigraphy for the diagnosis of extremity lymphedema: Current controversies regarding protocol, interpretation, and clinical application. J Surg Oncol. 2020;121(1):37-47. doi:10.1002/jso.25526
11. Narushima M, Yamamoto T, Ogata F, Yoshimatsu H, Mihara M, Koshima I. Indocyanine Green Lymphography Findings in Limb Lymphedema. J Reconstr Microsurg. 2016;32(1):72-79. doi:10.1055/s-0035-1564608
12. Kim G, Smith MP, Donohoe KJ, Johnson AR, Singhal D, Tsai LL. MRI staging of upper extremity secondary lymphedema: correlation with clinical measurements. Eur Radiol. 2020;30(8):4686-4694. doi:10.1007/s00330-020-06790-0
13. Kinmonth JB, Eustace PW. Lymph nodes and vessels in primary lymphoedema. Their relative importance in aetiology. Ann R Coll Surg Engl. 1976;58(4):278-284.
14. Soga S, Onishi F, Mikoshi A, Okuda S, Jinzaki M, Shinmoto H. Lower limb lymphedema staging based on magnetic resonance lymphangiography. Journal of Vascular Surgery: Venous and Lymphatic Disorders. Published online August 2021:S2213333X21003012. doi:10.1016/j.jvsv.2021.06.006
15. Executive Committee. The Diagnosis and Treatment of Peripheral Lymphedema: 2016 Consensus Document of the International Society of Lymphology. Lymphology. 2016;49(4):170-184.
Figures