2133
Iron Oxide Nanoparticle Magnetic Resonance Lymphangiography (ION-MRL): A New Technique for Imaging the Peripheral and Central Lymphatic System1Stanford University, Stanford, CA, United States
Synopsis
MR lymphangiography (MRL) utilizing the clinically available small molecular weight gadolinium contrast agents suffers from diffusion of contrast out of the lymphatic system and into the blood pool. We describe a modified MRL technique where a clinically available iron oxide nanoparticle (ferumoxytol) is used as the lymphatic contrast agent. The higher molecular weight of these nanoparticles results in retention of contrast within the lymphatic system. This simplifies peripheral lymphatic imaging by eliminating the need for vascular contamination suppression techniques and should allow better tracking of contrast through the central lymphatics following pedal injections.
Introduction
Gadolinium contrast enhanced MR lymphangiography (Gad-MRL) offers higher resolution than lymphoscintigraphy and is less invasive than conventional lymphangiograms. In evaluating the central lymphatics, Gad-MRL generally utilizes intranodal instillation of contrast in the groin.1,2 However, the procedure is technically complex as the injection requires ultrasound guided canulation of inguinal lymph nodes, and due to the prolonged needle insertion tenuously located within a lymph node hilum the entire procedure is generally performed under anesthesia.In evaluating the peripheral lymphatics, Gad-MRL utilizes intracutaneously installation of contrast in the feet or hands.3–5 However, the clinically available gadolinium contrast agents are suboptimal for lymphatic imaging as their size (~1nm, ~1kDa) allows for diffusion of contrast into the vasculature creating confusion in interpretation.3,6 Dual agent relaxation contrast (DARC) MRL successfully overcomes the issue of vascular contamination by co-administration of intravenous ferumoxytol to suppress vascular signal.7 However, DARC-MRL is relatively complicated compared to Gad-MRL, and the challenge remains of gadolinium contrast diffusing out of the lymphatic system and decreasing sensitivity for proximal pathology.8
Compared to gadolinium contrast agents, the iron oxide nanoparticle ferumoxytol is a more appropriate size (17-31 nm)9 for lymphatic imaging (similar in size to the agents utilized for lymphoscintigraphy). After intracutaneous injection ferumoxytol cannot readily diffuse into the blood vasculature, removing the need for vascular contamination suppression techniques and allowing lymphatic contrast to be tracked more proximally including through the central lymphatics. Given the relatively increased T2/T2* relaxivity of ferumoxytol compared to gadolinium agents, optimal imaging requires closer attention to minimizing echo times for T1-weighted imaging, and T1-weighted ultrashort echo time (UTE) sequences are advantageous. Though ferumoxytol has a black box warning for a risk of anaphylaxis with infusion, it has been demonstrated to be reasonably safe for off-label use as an MRI contrast agent10, and is free of gadolinium toxicity concerns. In this project, we explore appropriate injection conditions for iron oxide nanoparticle enhanced MR lymphangiography (ION-MRL) utilizing ferumoxytol and demonstrate preliminary examples of its use in clinical care.
Methods
Under an institutionally approved animal use committee protocol, a normal pig under anesthesia was evaluated with 3T imaging (GE 750). Contrast was injected subcutaneously in the hoofs, using 0.15 mmoles gadobenate dimeglumine or 6 mg ferumoxytol, in both cases diluted to 6 mL with saline. SPGR and UTE sequences (Table 1) were acquired after ferumoxytol injection.Human cases were performed as part of routine clinical care in 4 patients judged likely to benefit from ION-MRL after discussion with the patients. At our institution, all ferumoxytol use in MRI is monitored for 30 minutes following injection utilizing MRI compatible blood pressure cuffs and cardiac leads. Human MRI was performed on a 3T scanner (GE Signa Premier, or Signal Architect) using a combination of two anterior arrays (2x 30-channel GE AIR coils), and a 60-channel bed embedded posterior array. Sequences were similar to Table 1. 32-40 mg of ferumoxytol diluted to 16-20 mL in saline was split into 10 subcutaneous injections (5 injections per foot), with 1 min of massage applied to injection sites. Injection were given at the first and second interweb space, the anterolateral foot, the posterolateral foot and inferior to the medial maleous, in order to highlight the 4 major lymphosomes of the leg.11
Results
In the pig (Figure 1), R2* maps demonstrated R2* increase along ferumoxytol containing lymphatic channels. Ferumoxytol demonstrated improved visualization of cranial migration of contrast compared to gadobenate dimeglumine. Ferumoxytol concentration utilized was not overly concentrated for lymphatic vessel imaging. SPGR images on lymph nodes (where lymphatic contrast accumulates) demonstrated areas of signal loss compared to UTE sequences (Figure 1).ION-MRL was used in patient cases (Figures 2 and 3) performed due to concerns for central lymphatic obstruction due to thoracic duct injury (case 1 and 2) and venolymphatic malformation (case 4), and peripheral lymphedema due to metastatic adenopathy (case 3).
Discussion
Compared to Gad-MRL, ION-MRL can improve lymphatic imaging as the larger molecular size of ferumoxytol retains the contrast in the lymphatic system and prevents vascular contamination. This removes the need for vascular suppression techniques such as DARC-MRL and should allow better tracking of lymphatic contrast proximally. For evaluation of the central lymphatics, ION-MRL utilizing intracutaneous injections in the feet can replace the more procedurally complex process of intranodal administration of gadolinium contrast under general anesthesia or conscious sedation. In our patients evaluated with ION-MRL for central lymphatic abnormalities (cases 1, 2 and 4), the clinical question was answered without anesthesia. R2* based methods have been developed to allow quantitation of iron-oxide nanoparticles (e.g. ferumoxytol) over large concentration ranges 12–15; these can be used to quantitate lymphatic flow in ION-MRL. There is the potential for visible mild discoloration of the skin at injection sites lasting several months due to the reddish-brown color of ferumoxytol; this was not a concern to our patients but should be discussed with any patient for which ION-MRL is considered.Conclusion
We achieved diagnostic imaging of the central and peripheral lymphatics in several human cases using ION-MRL. Although we demonstrated a concentration of ferumoxytol that performed well in our cases, we did not evaluate whether this was an optimal concentration.Acknowledgements
No acknowledgement found.References
1. Krishnamurthy R, Hernandez A, Kavuk S, Annam A, Pimpalwar S. Imaging the Central Conducting Lymphatics: Initial Experience with Dynamic MR Lymphangiography. Radiology. 2015;274(3):871-878. doi:10.1148/radiol.14131399
2. Ramirez-Suarez KI, Tierradentro-Garcia LO, Smith CL, et al. Dynamic contrast-enhanced magnetic resonance lymphangiography. Pediatr Radiol. Published online April 8, 2021. doi:10.1007/s00247-021-05051-6
3. Lohrmann C, Foeldi E, Bartholomä JP, Langer M. Interstitial MR lymphangiography—A diagnostic imaging method for the evaluation of patients with clinically advanced stages of lymphedema. Acta Tropica. 2007;104(1):8-15. doi:10.1016/j.actatropica.2007.07.001
4. Lohrmann C, Foeldi E, Speck O, Langer M. High-Resolution MR Lymphangiography in Patients with Primary and Secondary Lymphedema. American Journal of Roentgenology. 2006;187(2):556-561. doi:10.2214/AJR.05.1750
5. Ruehm SG, Schroeder T, Debatin JF. Interstitial MR Lymphography with Gadoterate Meglumine: Initial Experience in Humans. Radiology. 2001;220(3):816-821. doi:10.1148/radiol.2203010090
6. Notohamiprodjo M, Baumeister RGH, Jakobs TF, et al. MR-lymphangiography at 3.0T—a feasibility study. Eur Radiol. 2009;19(11):2771-2778. doi:10.1007/s00330-009-1461-z
7. Maki JH, Neligan PC, Briller N, Mitsumori LM, Wilson GJ. Dark Blood Magnetic Resonance Lymphangiography Using Dual-Agent Relaxivity Contrast (DARC-MRL): A Novel Method Combining Gadolinium and Iron Contrast Agents. Current Problems in Diagnostic Radiology. 2016;45(3):174-179. doi:10.1067/j.cpradiol.2015.08.003
8. Lower limb lymphedema staging based on magnetic resonance lymphangiography - ClinicalKey. Accessed October 1, 2021. https://www.clinicalkey.com/#!/content/playContent/1-s2.0-S2213333X21003012?scrollTo=%23bib16
9. DailyMed - FERAHEME- ferumoxytol injection. Accessed September 23, 2021. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=32b0e320-a739-11dc-a704-0002a5d5c51b
10. Nguyen KL, Yoshida T, Kathuria-Prakash N, et al. Multicenter Safety and Practice for Off-Label Diagnostic Use of Ferumoxytol in MRI. Radiology. 2019;293(3):554-564. doi:10.1148/radiol.2019190477
11. Shinaoka A, Koshimune S, Suami H, et al. Lower-Limb Lymphatic Drainage Pathways and Lymph Nodes: A CT Lymphangiography Cadaver Study. Radiology. 2020;294(1):223-229. doi:10.1148/radiol.2019191169
12. Liu W, Dahnke H, Rahmer J, Jordan EK, Frank JA. Ultrashort T2* relaxometry for quantitation of highly concentrated superparamagnetic iron oxide (SPIO) nanoparticle labeled cells. Magn Reson Med. 2009;61(4):761-766. doi:10.1002/mrm.21923
13. Magnitsky S, Zhang J, Idiyatullin D, et al. Positive contrast from cells labeled with iron oxide nanoparticles: Quantitation of imaging data. Magn Reson Med. 2017;78(5):1900-1910. doi:10.1002/mrm.26585
14. Sharma SD, Fischer R, Schoennagel BP, et al. MRI-based quantitative susceptibility mapping (QSM) and R2* mapping of liver iron overload: Comparison with SQUID-based biomagnetic liver susceptometry. Magn Reson Med. 2017;78(1):264-270. doi:10.1002/mrm.26358
15. Vasanawala SS, Yu H, Shimakawa A, Jeng M, Brittain JH. Estimation of liver T₂ in transfusion-related iron overload in patients with weighted least squares T₂ IDEAL. Magn Reson Med. 2012;67(1):183-190. doi:10.1002/mrm.22986
Figures
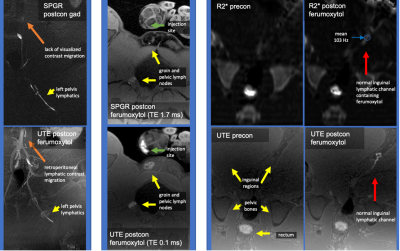
Figure 1: Normal Pig Gad-MRL and ION-MRL
Left column gadobenate dimeglumine (top) and ferumoxytol MRL (bottom) injections into the left hoof, note improved visualization of contrast migration with ferumoxytol (orange arrows). Middle column compares SPGR versus UTE sequences after right hoof ferumoxytol injection, note areas of signal loss with longer TE sequence. Right columns SPGR R2* maps (top) and UTE T1w images (bottom) pre and post ferumoxytol administration into the left hoof, note the R2* increase (T2* decrease to 8ms) along ferumoxytol containing channels.
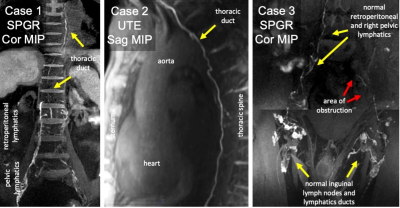
Figure 2: ION-MRL in Humans
Case 1: Chronic effusions, suspected thoracic duct obstruction, ferumoxytol used due to renal failure. The thoracic duct is continuous. 3D SPGR Dixon TE 1.7 ms (effective). Case 2: Paraspinal mass resection with suspected thoracic duct transection. Sagittal MIP demonstrates thoracic duct continuity. UTE SPAIR TE 0.1ms. Case 3: Prostate cancer with left lower extremity edema, ferumoxytol used due to patient concerns regarding gadolinium. Coronal MIP images demonstrate poor transit of contrast in the left pelvis. 3D SPGR Dixon TE 2.8ms (effective).
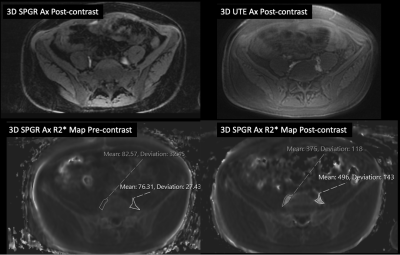
Figure 3: Quantification of R2* in ION-MRL
Case 4: Post contrast imaging after ferumoxytol injected intradermally into 8 sites in both feet. SPGR and UTE images demonstrate ferumoxytol uptake in the retroperitoneal lymphatics. Pre-contrast R2* measures 83 and 76 sec-1 for the right and left retroperitoneal lymphatics respectively. Postcontrast R2* measures 375 and 496 sec-1 for the right and left retroperitoneal lymphatics respectively demonstrating the quantitative utility of R2* in ION-MRL.
