2127
Real-time quality assurance for volumetric motion estimation during MR-guided radiotherapy1Department of Radiotherapy, Computational Imaging Group for MR therapy & Diagnostics, University Medical Center Utrecht, Utrecht, Netherlands
Synopsis
Respiratory motion during radiotherapy decreases the time-efficiency of treatments. The MR-Linac can potentially improve this with real-time 3D MR-based motion estimates. Ideally, these estimates should be accompanied with estimation uncertainty to ensure patient safety and spare organs-at-risk during bulk motion or breathing pattern change, but this is typically not provided by state-of-the-art methods. In this work we extend a previously proposed probabilistic framework for real-time motion and uncertainty estimation with a rejection criterion based on the estimation uncertainty. Results show that the proposed rejection criterion preserves low end-point-errors and rejects erroneous motion estimates during bulk motion and breathing pattern changes.
Introduction
Respiratory and physiological motion during abdominothoracic radiotherapy increases the uncertainty in the tumor's location. A typical solution is respiratory-gated irradiation, which reduces the time-efficiency of radiotherapy treatments. The MR-Linac[1] could improve this efficiency by real-time radiation adaption given real-time MRI-based estimates of tumor and organs-at-risk locations.This introduces the challenge of estimating high-quality 3D motion-field at >5Hz[2]. This is typically tackled by employing a normal breathing motion model[3,4], but motion estimates during e.g. bulk motion or breathing pattern changes could potentially be erroneous. To assure patient safety during such events, ideally also a measure of confidence should be provided; the treatment could be temporarily halted during high uncertainty.
Previous work[5] demonstrated the proof-of-concept of joint real-time 3D motion-field and uncertainty estimation from few MR-data by exploiting a motion model[3,4] for normal breathing. Preliminary results indicated increased uncertainty during unexpected motion for which the motion model was not valid and thus inapplicable.
In this work, we extend and extensively validate our framework with real-time quality assurance based on this uncertainty. Moreover, the extended framework is validated in-silico, and its robustness assessed in-vivo on MR-Linac data of five volunteers, considering several breathing patterns, and bulk motion.
Theory
Background on Gaussian ProcessesOur previous work[5] employs a probabilistic machine learning method called Gaussian Processes[6] (GPs) to jointly estimate 3D motion-fields uncertainties from ultra-short acquisitions. GPs are frequently applied to regression; given training samples $$$\mathcal{T}$$$ including observed function values and measurement locations, GPs yield[6] a rapidly computable analytical expression for the posterior mean and standard deviation of (unobserved) function values $$$Y_\mathcal{Q}$$$ at $$$X_\mathcal{Q}$$$.
We applied[5] GPs to multi-dimensional regression between a compressed representation of mutually orthogonal readouts, and representation coefficients of a 3D motion model (Fig. 1). Patient-specific training is performed within seconds on three minutes of free-breathing data. Real-time inference with three readouts results in 3D motion-field and uncertainty estimation at 67Hz.
Rejection criterion based on posterior uncertainty
Bulk motion or breathing pattern changes possibly result in erroneous estimates due to model imperfection. Preliminary results indicated an increased posterior uncertainty during such unfavourable motion. To assure patient safety in this scenario, we propose a rejection threshold as the 99th percentile of posterior uncertainties during normal breathing. Dynamics with posterior uncertainty exceeding the threshold are flagged, indicating the radiation should be temporarily halted.
Methods
Data acquisition/simulationTo facilitate 3D motion-field estimation and GP training, data is acquired/simulated with a golden-mean 3D radial (GM3DR) trajectory, interleaved with self-navigation (SN) readouts along FH,AP,LR[3,5]. In-silico multi-channel data (SNR=60) is generated with XCAT[7,8,9]. In-vivo data is acquired from five healthy volunteers on a Unity MR-Linac (Elekta AB, Sweden), using a spoiled gradient-echo, TR/TE/FA=4.8/1.8/20.
In-silico validation
Four different breathing patterns are simulated: normal (chest+abdomen), abdominal-only, chest-only, and amplitude drifts. The framework is trained on normal breathing, and tested on all breathing patterns. End-point-errors (EPEs) with ground-truth XCAT motion-fields are computed on a liver lesion, thereby comparing EPEs before and after rejection.
In-vivo experiments
The robustness of the proposed extended framework is assessed in-vivo on five healthy volunteers. Two volunteers (#3,5) were instructed to change breathing pattern and perform bulk motion at specific timestamps (Fig. 5), the rest was not instructed. The motion estimates are validated with projections along FH obtained with a 1D-FFT.
Results
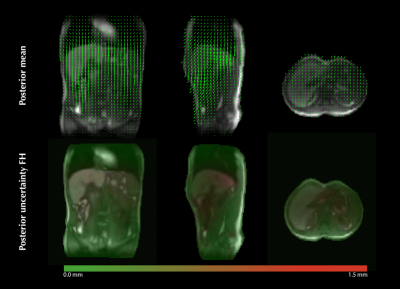
Figure 2 shows the in-silico validation. During 75% of normal breathing the EPEs are below 1mm, with a maximum of 1.5mm. For other breathing patterns the EPEs increase due to motion model imperfection. The boxplots show strong statistical evidence (p<=0.05) of the rejection criterion's effectivity to preserve low EPEs similar to normal breathing, i.e. to correctly reject inaccurate estimates.Figure 3 shows an example of an estimated 3D motion-field and uncertainty map during normal breathing.
Figure 4 shows the feasibility of in-vivo inference. Bulk motion and breathing pattern changes in volunteer 3 and 5 (4B, column 2+3), and large breathing amplitudes for other volunteers (4A, column 3) are accompanied with increased estimation uncertainty.
Figure 5 shows the in-vivo validation of the rejection criterion and the correspondence between uncertainty and unexpected motion. Flagged (red) motion estimates (blue) largely correspond with visually different FH projections. This indicates that the rejection criterion flags dynamics acquired during unexpected motion, for which the radiation therapy should ideally be temporarily halted in practice because of potentially erroneous motion estimates.
Discussion & Conclusion
We presented and validated a method for real-time motion estimation with quality assurance for MR-guided radiotherapy, based on motion estimation uncertainty from our previously introduced probabilistic framework. The method allows to reject potentially erroneous motion estimates due inapplicability of the motion model. Although paramount to ensure patient safety during treatments, this is not yet available in state-of-the-art methods.The accuracy and robustness of the extended framework is assessed in practical settings under different breathing patterns, showing the feasibility of high-speed motion inference, and strong statistical evidence of the effectivity of the proposed rejection criterion to preserve low end-point-errors.
We envision an application in MR-guided radiotherapy, where the extended framework is employed for real-time 3D motion-field estimation and flags dynamics for which radiation should be temporarily halted. Additionally, the motion model could be updated in parallel to continue treatments when many subsequent samples are rejected.
Acknowledgements
This research is funded by the Netherlands Organisation for Scientific Research, domain Applied and Engineering Sciences, Grant number: 15115.
References
- Raaymakers, BW, et al. "Integrating a 1.5 T MRI scanner with a 6 MV accelerator: proof of concept." Physics in Medicine & Biology, 2009.
-
Keall, PJ, et al. "The management of respiratory motion in radiation oncology report of AAPM Task Group 76 a." Medical physics, 2006.
- Huttinga, NRF, et al. "Real-time non-rigid 3D respiratory motion estimation for MR-guided radiotherapy using MR-MOTUS", IEEE Trans. Med. Imag., 2021
- Huttinga, NRF, et al. "Nonrigid 3D motion estimation at high temporal resolution from prospectively undersampled k-space data using low-rank MR-MOTUS", Magn. Reson. Med., 2020
- Huttinga, NRF, et al. "Joint 3D motion-field and uncertainty estimation at 67Hz on an MR-LINAC." Proc. Intl. Soc. Mag. Reson. Med. Vol. 29, 2021.
- Rasmussen, CE. “Gaussian processes in machine learning,” Summer School on Machine Learning, 2003.
- Segars, WP, et al. “4D XCAT phantom for multimodality imaging research,” Medical Physics, 2010.
- Barnett, AH, et al. “A parallel nonuniform fast Fourier transform library based on an ‘exponential of semicircle’ kernel,” SIAM J. Sci. Comput., 2019.
- Uecker, M, et al. "Berkeley advanced reconstruction toolbox." Proc. Intl. Soc. Mag. Reson. Med. Vol. 23, 2015.
- Zachiu, C, et al. "An improved optical flow tracking technique for real-time MR-guided beam therapies in moving organs," Phys. Med. Biol., 2015
Figures