2124
Visualizing uterine fibroid perfusion after MR-HIFU ablations using T2-corrected IVIM and deep learning-based fitting – an explorative study.1Radiology, Isala Zwolle, Zwolle, Netherlands, 2Image Sciences Institute, Imaging & Oncology Division, University Medical Center Utrecht, Utrecht, Netherlands, 3Faculty of Medicine and University Hospital of Cologne, Institute of Diagnostic and Interventional Radiology, University of Cologne, Cologne, Germany, 4High Tech Campus, Philips Research Eindhoven, Eindhoven, Netherlands
Synopsis
Blood volume fraction maps derived from diffusion weighted imaging using intravoxel incoherent motion (IVIM) modeling may be an alternative to contrast-enhanced imaging for visualization of local uterine fibroid perfusion, during magnetic resonance-guided high intensity focused ultrasound (MR-HIFU) ablation procedures. In this study, blood volume fraction maps calculated with a T2-corrected IVIM model were compared to T2-uncorrected parameter maps using two fitting techniques. Based on general inspection, T2-corrected parameter maps from deep learning-based fitting were visually the most appealing. A preliminary evaluation showed a linear association between NPVs delineated on blood volume fraction maps and gadolinium contrast-enhanced T1w scans.
Introduction
Assessment of technical success after magnetic resonance-guided high intensity focused ultrasound (MR-HIFU) ablations of uterine fibroids (UFs) is largely based on imaging of tissue perfusion. A large non-perfused volume (NPV) relative to the total UF volume, i.e. NPV-ratio, is an important predictor of clinical outcomes1–7. To assess the NPV after MR-HIFU, gadolinium-based contrast-enhanced (CE) imaging is standardly acquired. Due to safety concerns and dose constraints, CE scans cannot be repeated during MR-HIFU8,9. Therefore, efforts have been made to conceive alternative methods for visualization of UF perfusion, including subtraction of parameters from diffusion weighted imaging (DWI) using intravoxel incoherent motion (IVIM) modeling:$$I(b) = I(0)(fe^{-bD^\ast}+(1-f)e^{-bD})$$ Equation 1
where $$$I$$$ is the signal intensity at diffusion weighting $$$b$$$ (s/mm2), $$$D$$$ the diffusion coefficient, $$$D^{\ast}$$$ the pseudo-diffusion (perfusion) coefficient, and $$$f$$$ the blood volume fraction. It has been suggested that $$$f$$$ is related to perfusion of UF tissue, and could therefore be an important biomarker to predict MR-HIFU therapy success10. Since T2 values differ between both compartments of eq.1 (muscle tissue: 44ms, blood: 290ms11), eq.1 can be extended to a T2-corrected IVIM-model that incorporates signal loss due to spin-spin relaxation at echo time TE12–14:
$$I(b) = I_0(e^{\frac{-TE}{T2_{blood}}}*f_ce^{-bD_c^\ast}+e^{\frac{-TE}{T2_{tissue}}}*(1-f_c)e^{-bD_c})$$ Equation 2
where $$$I_0$$$ is the overall signal intensity, and $$$f_c$$$, $$$D_c$$$ and $$$D_c^{\ast}$$$ the respective T2-corrected equivalents of $$$f$$$, $$$D$$$ and $$$D^{\ast}$$$. Qu et al. (2018) have advocated the need of T2-correction when applying IVIM to characterize UFs, and suggested that fc may serve as non-contrast biomarker to gather information about local UF perfusion, but this needs further investigation13. Our aim was to compare the blood volume fraction with and without T2-correction quantitatively. In addition, the conventional least squares regression fitting method15 was compared to an alternative deep learning-based method16. It has been proposed that a deep learning-based fitting method yields faster fitting times and improved visual parameter maps16–18, and could therefore be beneficial when applying IVIM intraprocedurally to assess NPVs. Finally, a preliminary qualitative comparison between NPVs delineated on $$$f$$$-parameter maps and CE-T1w scans was performed.
Methods
In this retrospective study, data from fifty-six women who underwent an MR-HIFU treatment of symptomatic UFs was used. The DWI scans were acquired directly after the HIFU procedure on a 1.5T MR-system (Achieva, Philips Healthcare, Best, The Netherlands), using a fat-suppressed multi-slice single-shot spin echo-echo planar sequence (TR/TE = 1352ms/65ms, acquired voxel size: 2.50x4.36x6.00 mm3, reconstructed voxel size: 0.89x0.90x6.00 mm3), at different diffusion strengths (b-values 0, 50, 100, 200, 400, 600 and 800 s/mm2). T2-maps were calculated on the MR console, derived from a multi-spin-echo sequence (TR/TE = 2000ms/0-240ms with 20ms echo-spacing, acquired voxel size: 2.50x2.81x7.00mm3, reconstructed voxel size: 1.76x1.76x7.00mm3). Acquired T2-maps were resampled using linear interpolation to the DWI coordinate system. Furthermore, anatomical T2w and gadolinium CE-T1w scans were acquired.Delineations of UFs and NPVs were drawn on the paired anatomical T2w and CE-T1w scans by two researchers trained and supervised by radiologists. First, eq.1 and eq.2 were fit to DWI scans to acquire T2-uncorrected and -corrected parameter maps respectively using conventional least squares regression. Second, a neural net existing of five hidden layers based on the implementation of Barbieri et al. (2020)16 was deployed as fitting method. DWI voxels containing b-values and T2-values within UFs from five consecutive patients were used as input to train the neural net.
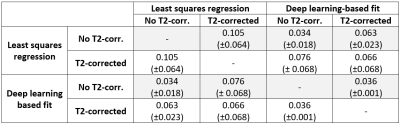
Final blood volume fraction maps derived from eq.1 and eq.2 using least squares- and deep learning-based fitting were quantitatively compared by calculating the mean absolute error (MAE) within the UF. NPVs delineated on least-squares T2-uncorrected $$$f$$$-parameter maps by a trained researcher were compared to NPVs derived from CE-T1w scans. P-values below .05 were considered statistically significant.
Results
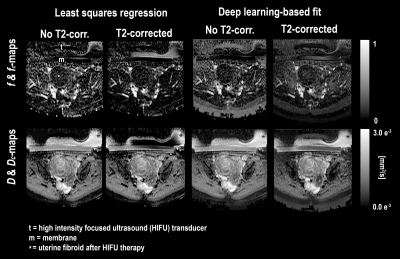
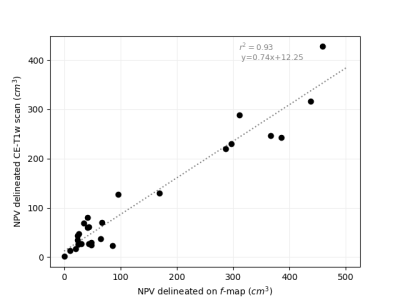
Parameter maps could be successfully produced using conventional and deep learning-based fitting method using eq.1 and eq.2. Representative examples of $$$f$$$-,$$$f_c$$$-maps and $$$D$$$-,$$$D_c$$$-maps are depicted in Figure 1. Upon general inspection, the appearance of $$$f_c$$$-maps acquired with deep learning-based fitting was the most visually appealing blood volume fraction map. For least squares fitting, the average $$$f$$$ and $$$f_c$$$ values where 0.180 (±0.052) and 0.112 (±0.029) (P<0.001), and for deep learning-based fitting 0.155 (±0.045) and 0.145 (±0.046) (P=0.247), respectively. MAEs are listed in Table 1. A positive association was found between NPVs delineated on $$$f$$$-parameter maps and CE-T1w scans (P<0.001, Figure 2).Discussion
Creating blood volume fraction maps from DWI with IVIM could be a contrast agent-free method to visualize the UF tissue perfusion status in the context of MR-HIFU treatments. Here, it was found that the blood volume fraction was significantly lower in T2-corrected parameter maps after using a least squares regression fitting method, in accordance with Qu et al. (2018)13. This decrease was not found after applying deep learning-based fitting, while blood volume fraction maps seemed to improve after T2-correction, based on general visual inspection. Although an additional examination by multiple radiology experts is necessary, our preliminary evaluation showed a large linear association between NPVs delineated on blood volume fraction maps and CE-T1w scans.Conclusion
Blood volume fraction derived from DWI using IVIM modeling show potential as contrast-agent free method for visualization of the UF perfusion status after ablation. Using a T2-corrected deep learning-based fitting method for this purpose seems favorable.Acknowledgements
NAReferences
1. Verpalen, I. M. et al. Magnetic resonance-high intensity focused ultrasound (MR-HIFU) therapy of symptomatic uterine fibroids with unrestrictive treatment protocols: A systematic review and meta-analysis. Eur. J. Radiol. 120, 108700 (2019).
2. Fennessy, F. M. et al. Uterine leiomyomas: MR imaging-guided focused ultrasound surgery - Results of different treatment protocols. Radiology 243, 885–893 (2007).
3. Park, M. J., Kim, Y. S., Rhim, H. & Lim, H. K. Safety and therapeutic efficacy of complete or near-complete ablation of symptomatic uterine fibroid tumors by MR imaging-guided high-intensity focused US Therapy. J. Vasc. Interv. Radiol. 25, 231–239 (2014).
4. Al Hilli, M. M. & Stewart, E. A. Magnetic resonance-guided focused ultrasound surgery. Seminars in Reproductive Medicine vol. 28 242–249 (2010).
5. Stewart, E. A. et al. Sustained relief of leiomyoma symptoms by using focused ultrasound surgery. Obstet. Gynecol. 110, 279–287 (2007).
6. Verpalen, I. M. et al. The Focused Ultrasound Myoma Outcome Study (FUMOS); a retrospective cohort study on long-term outcomes of MR-HIFU therapy. Eur. Radiol. 30, 2473–2482 (2020).
7. Keserci, B. & Duc, N. M. The role of T1 perfusion-based classification in magnetic resonance-guided high-intensity focused ultrasound ablation of uterine fibroids. Eur. Radiol. 27, 5299–5308 (2017).
8. Hijnen, N. M. et al. The magnetic susceptibility effect of gadolinium-based contrast agents on PRFS-based MR thermometry during thermal interventions. J. Ther. Ultrasound 1, 1–9 (2013).
9. Hijnen, N. M., Elevelt, A. & Grüll, H. Stability and trapping of magnetic resonance imaging contrast agents during high-intensity focused ultrasound ablation therapy. Invest. Radiol. 48, 517–524 (2013).
10. Ikink, M. E. et al. Diffusion-weighted magnetic resonance imaging using different b-value combinations for the evaluation of treatment results after volumetric MR-guided high-intensity focused ultrasound ablation of uterine fibroids. Eur. Radiol. 24, 2118–2127 (2014).
11. Stanisz, G. J. et al. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magn. Reson. Med. 54, 507–512 (2005).
12. Lemke, A., Laun, F. B., Simon, D., Stieltjes, B. & Schad, L. R. An in vivo verification of the intravoxel incoherent motion effect in diffusion-weighted imaging of the abdomen. Magn. Reson. Med. 64, 1580–1585 (2010).
13. Qu, F., Hor, P.-H., Fischer, J. & Muthupillai, R. Tissue characterization of uterine fibroids with an intravoxel incoherent motion model: The need for T2 correction. J. Magn. Reson. Imaging 48, 994–1001 (2018).
14. Jerome, N. P. et al. Extended T2-IVIM model for correction of TE dependence of pseudo-diffusion volume fraction in clinical diffusion-weighted magnetic resonance imaging. Phys. Med. Biol. 61, N667 (2016).
15. While, P. T. A comparative simulation study of bayesian fitting approaches to intravoxel incoherent motion modeling in diffusion-weighted MRI. Magn. Reson. Med. 78, 2373–2387 (2017).
16. Barbieri, S., Gurney‐Champion, O. J., Klaassen, R. & Thoeny, H. C. Deep learning how to fit an intravoxel incoherent motion model to diffusion‐weighted MRI. Magn. Reson. Med. 83, 312–321 (2020).
17. Koopman, T. et al. Repeatability of IVIM biomarkers from diffusion‐weighted MRI in head and neck: Bayesian probability versus neural network. Magn. Reson. Med. 85, 3394 (2021).
18. Kaandorp, M. P. T. et al. Improved unsupervised physics-informed deep learning for intravoxel incoherent motion modeling and evaluation in pancreatic cancer patients. Magn. Reson. Med. 86, 2250–2265 (2020).
Figures