2123
Olfactory Signal processing in functional MRI oddball task1Department of Radiology, Pennsylvania State University College of Medicine, Hershey, PA, United States, 2Department of Psychiatry, Pennsylvania State University College of Medicine, Hershey, PA, United States, 3Department of Molecular Biology, Pennsylvania State University College of Medicine, Hershey, PA, United States, 4Department of Neurosurgery, Pennsylvania State University College of Medicine, Hershey, PA, United States
Synopsis
Odor-identification is thought to be subserved by a distributed brain network that includes both the medial and inferior temporal lobes, still, its neural substrate remains poorly understood. Odor identification deficits are one of the hallmark symptoms of early Alzheimer’s disease (AD). Here, we propose the development of an oddball olfactory fMRI paradigm to establish the neural basis of odor-identification in healthy subjects. This task may provide a basis for establishing relationships between olfactory deficits, neurodegeneration, and memory impairment for current AD research.
Introduction
Olfactory function, particularly, odor-identification, is affected in mild cognitive impairment (MCI) and preclinical AD1. Unfortunately, we do not yet know the brain basis of odor-identification. Such information is necessary to relate AD neurodegeneration to specific functional deficits in olfaction. As a first step, we propose the development of an olfactory functional magnetic resonance imaging (fMRI) oddball detection task for healthy adults in order to identify the neural substrate that supports odor-identification.Methods
Olfactory fMRISeventeen healthy young subjects (age < 30 yrs.) completed four identical runs of the oddball detection olfactory task (Figure 1) on a Siemens 3.0 Tesla clinical MRI system (Prisma Fit, Siemens Medical Solutions, Inc., Erlangen, Germany) with a 20-channel head coil. The study was approved by the Penn State College of Medicine IRB and all volunteers provided written informed consent. The oddball task required subjects to press a button with the right index finger to identify the lavender odorant: the oddball. During fMRI scanning, subjects were positioned in a supine position in a dark environment with their heads elevated 10o-15o from the horizontal level. The subjects’ respiration and sniffing patterns were also monitored to exclude irregular respiration volume and rate changes. A T2*-weighted EPI sequence was used for fMRI image acquisition with the following parameters: TR / TE / FA = 2000 ms / 30 ms / 70o; FOV = 220 mm x 220 mm; image resolution = 2.75 mm × 2.75 mm; 36 slices with a thickness of 4 mm. A T1-weighted MPRAGE image was also acquired for anatomical overlay.
Data Processing and Analysis
The fMRI data were normalized to the Montreal Neurological Institute brain template. SPM12 was used to perform fMRI data analyses to identify locations of olfactory activation.
Results and Discussion
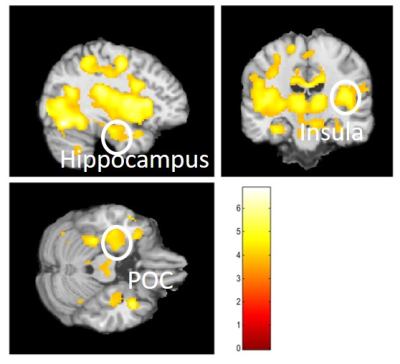
Our oddball olfactory fMRI paradigm identified a brain network consisting of the primary olfactory cortex (POC), insula, hippocampus, and other brain structures in the medial temporal lobe (MTL) and inferior temporal (IT) cortex (Figure 2). These brain regions are known to be affected by neurodegeneration2, 3. The proposed paradigm is novel because it has the following features: a dynamic range in odor-identification for testing people with normal cognition and MCI, and the task is modeled after olfactory behavioral tests such as UPSIT and OLFACT. Therefore, the fMRI activation during these tasks can be directly correlated to task performance scores and UPSIT/OLFACT scores4, 5. Odor-identification is significantly reduced in early AD patients relative to age matched controls. Our paradigm provides the basis for the development of a comprehensive neurocognitive model for odor-identification, which can be applied to broader translational studies on olfactory function, aging, and neurological disease.Conclusion
The significant knowledge gaps regarding the neural bases of odor-identification and their impairment impedes the development of functional imaging markers for the evaluation and diagnosis of AD. Our results show the advantages of investigating odor-identification using the oddball olfactory fMRI paradigm. This paradigm has the capacity to provide a foundation for improving upon, and identifying, the olfactory impairments common to specific neurological diseases, such as AD.Acknowledgements
This study was partly supported by a grants from the National Institute on Aging grants (1R01AG070088-01A1 and1R21AG064486) and Department of Radiology, Pennsylvania State University College of Medicine. Authors are thankful to the MRI Core Facility staff for the support on study protocol development, data acquisition, data processing and data analysis in this study.
References
[1]. Murphy C. Olfactory and other sensory impairments in Alzheimer disease. Nat Rev Neurol. Jan 2019;15(1):11-24.
[2]. Price JL. Olfactory system. In P. G (Ed.), The Human Nervous System (pp. 979-998) : San Diego, CA: Academic Press; 1990.[3]. Vasavada MM, Wang J, Eslinger PJ, et al. Olfactory cortex degeneration in Alzheimer's disease and mild cognitive impairment. Journal of Alzheimer's Disease. 2015;45(3):947-958.[4]. Doty RL, Shaman P, Kimmelman CP, Dann MS. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. The Laryngoscope. 1984;94(2):176-178.[5]. Zhang Z, Zhang B, Wang X, et al. Altered odor-induced brain activity as an early manifestation of cognitive decline in patients with type 2 diabetes. Diabetes. 2018;67(5):994-1006.
Figures
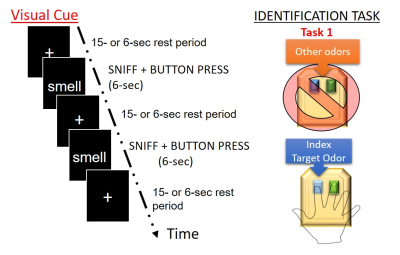
Figure 1. A visual representation of what subjects will be shown in the scanner, including what buttons will be pressed for each specified event