2119
Understanding the effect of noise on cognition using attention framework Navon task1Department of NMR and MRI Facility, All India Institute of Medical Sciences, New Delhi, India
Synopsis
Cognitive studies using functional imaging are inherently associated with nonstimulant noise in MRI. Dissociating the noise aspect in functional imaging from cognitive traits is crucial in delineating auditory cognition. This study explores the association of noise in auditory cognition studies. we designed a functional Navon task paradigm with introduction of pink noise characteristics modelled as part of its memory phase. Variation of noise induced the cognitive masking associated with characteristics of stimuli across the local and global suppression.
Introduction
Functional magnetic resonance imaging (fMRI) has become an utmost technique for cognitive research and to understand the cognitive to brain network lateralization. Benefits of the techniques have superseded its acoustic sensory load associated with MRI. Acoustic noise can interfere with cognitive processes due to sensitivity reduction, execution challenge due to stimulus degradation and attentional distraction1. In the present study, we studied the attentional framework associated with working memory due to noise of MR scanner (in addition to stimulus noise).Method
We designed an fMRI-based alphabet Navon task with continuous noise (scanner) and deviant noise (pink noise) using paradigm presentation software (Superlab 5, Cedrus Inc, USA). A pink noise stimulus was supplied through a waveguide and amplifier to remain audible in contrast to scanner noise overlapping the memory phase. Healthy volunteers (n=32) underwent fMRI study for Navon module in a 3T MR scanner (Ingenia 3T, M/s Philips). To understand functional changes due to variation of continuous and deviant noise, a generalized Psychophysiological Interactions (gPPI) model using local and global perception suppression condition was computed. Additionally, we estimated functional connectivity cluster for cognition and its interaction with noise conditions across local and global states using Multivariate Correlation (MVPA).Result
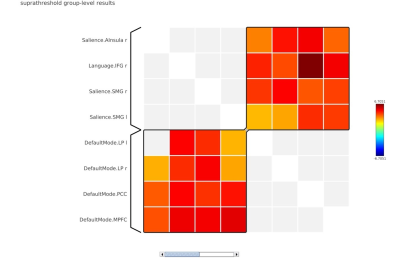
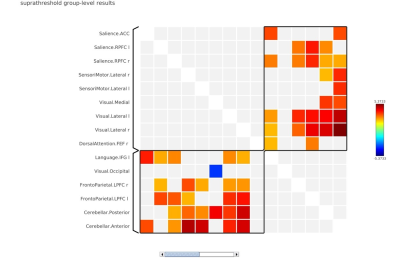
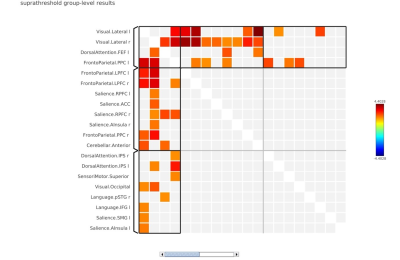
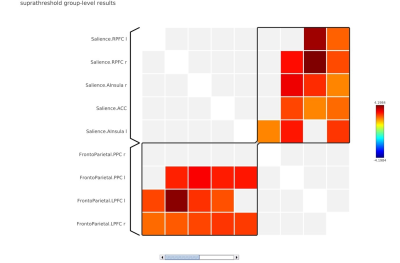
In local perception suppression task, memory cognition (with continuous noise only) revealed default mode-lateral posterior parietal cortex (LPP) (r/l), medial prefrontal cortex (MPFC) and posterior cingulate (PCC) with a positive correlation interaction with saliency-aInsula(r), SMG (r/l) and language-inferior frontal gyrus (IFG) (MVPA cluster statistics of F=18.91, p<0.001, Figure 1). In deviant condition lateral frontoparietal cortex (LPFC) and Language-IFG exhibited a positive interaction with saliency-right prefrontal Cortex (RPFC) and anterior cingulate cortex (ACC) network (MVPA cluster statistics of F=28.64, p<0.001, Figure 2). In global perception suppression task, memory cognition (with continuous noise only) revealed a positive interaction of Dorsal attention-frontal eye fields (FEF) (l) with language (MVPA cluster statistics of F=10.39, p<0.01, Figure 3) and saliency RPFC(l) positive interaction with frontoparietal-posterior parietal cortex (PPC)(l) and Dorsal attention-FEF(l) (MVPA cluster statistics of F=9.69, p<0.01, Figure 3). In deviant condition, frontoparietal-LPFC(r/l) and frontoparietal-PPC(r/l) exhibited a positive interaction with saliency-RPFC(r/l), aInsula(r/l) and ACC (MVPA cluster statistics of F=10.98, p<0.01, Figure 4). Visual network interaction was observed with frontoparietal in local suppression with deviant noise and with dorsal attention in global suppression with continuous noise.Discussion
In Navon task, during local suppression of information, the deviant sound induced cognitive interaction was representative of visual, executive (saliency) and modulating attention network (Figure 2). During global suppression of information, deviant sound induced cognitive interaction was representative of executive (Saliency) and attention network (Figure 4). Such aspect of deviant noise effect in cognitive connectivity for global condition represent auditory perception masking associated process in high cognitive load setting resulting in suppression of visual field aspect, that is representative characteristics of Navon task (jack). During scanner noise, functional connectome revealed the default mode and saliency interaction in local suppression (Figure 1). Interaction was more dispersed in global suppression, wherein deviant noise masking effect can be clearly understood. Research suggests that noise may facilitate auditory working memory performance via stochastic resonance, where induced noise will either improve the auditory pathway interaction to stimuli or deteriorate depending upon cognitive as a function of noise induced level2,3. In current task the focus was to understand the additional aspect associated with resonance of sound, attentional loading and its underlying changes. As observed in the study, attentional framework is important in performance and cognitive changes estimate in respect to exposure of sound4.Conclusion
Effect of noise on cognition influences the neural response via auditory pathway interaction1. Current work highlights the importance of delineating the acoustics exposure to cognitive process and further bifurcating the loading characteristics associated with working memory. As auditory cognition is interlinked with attention-perception framework, such underling changes due to attention load challenge during induced noise can be misinterpreted in cognitive studies with auditory cognition.Acknowledgements
The funding from Life Sciences Research Board (LSRB), Ministry of defence, Government of India is duly acknowledged (vide grant No. LSRB-295/PEE&BS/2017)References
1. Peelle JE. Methodological challenges and solutions in auditory functional magnetic resonance imaging. Front Neurosci. 2014;8:253. doi: 10.3389/fnins.2014.00253.
2. Othman E, Yusoff AN, Mohamad M, et al. Low intensity white noise improves performance in auditory working memory task: An fMRI study. Heliyon. 2019;5(9):e02444. doi: 10.1016/j.heliyon.2019.e02444.
3. Schoffelen JM, Hultén A, Lam N, Marquand AF, Uddén J, Hagoort P. Frequency-specific directed interactions in the human brain network for language. Proc Natl Acad Sci U S A. 2017;114(30):8083-8088. doi: 10.1073/pnas.1703155114.
4. Herweg NA, Bunzeck N. Differential effects of white noise in cognitive and perceptual tasks. Front Psychol. 2015;6:1639. doi: 10.3389/fpsyg.2015.01639.
Figures