2118
Temporal properties of fast BOLD fMRI responses in veins and parenchyma1Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Department of Biomedical Engineering, Boston University, Boston, MA, United States, 3Department of Radiology, Harvard Medical School, Boston, MA, United States, 4Department of Neuroscience, University of Copenhagen, Copenhagen, Denmark, 5Harvard-MIT Division of Life Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States
Synopsis
Blood-oxygen-level-dependent (BOLD) signals can produce much faster (>0.2Hz) responses than predicted by canonical hemodynamic response models. The timing of hemodynamic responses depends on vascular anatomy, and whether these fast responses differ in veins and parenchyma is unknown. Linear models predict that with faster stimuli, venous responses should attenuate more than the parenchyma. We tested this hypothesis by imaging visual responses at high spatial resolution with 7T fMRI. We found that both veins and parenchyma produce larger fMRI responses to fast stimuli than predicted by linear models, suggesting that faster stimuli produce narrower hemodynamic responses in both compartments.
Introduction
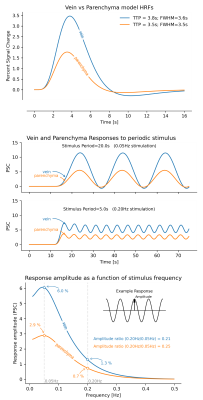
Understanding the spatiotemporal properties of the BOLD signal is critical for interpreting fMRI studies. BOLD responses are most pronounced in large draining veins, away from the site of activation, and these venous responses are delayed relative to the parenchyma (1). The different temporal dynamics in veins and parenchyma can be modelled by assuming compartment-specific hemodynamic response functions (HRFs), with a wider and slower HRF in the veins (2). This difference in the speed of HRFs suggest that veins could have different responses to rapid stimulus paradigms: broader and slower HRFs attenuate fast activity more strongly than narrower HRFs. These models therefore predict that using sufficiently fast stimuli, BOLD responses should become less pronounced in veins (Fig. 1). However, since HRFs also depend on stimulus duration, whether this prediction holds in the context of fast stimulus paradigms is unknown.Here we investigated whether veins and parenchyma exhibit different temporal properties in response to fast stimuli. We studied responses in the primary visual cortex, induced by a flickering checkerboard stimulus with luminance contrast oscillating at different frequencies (0.05Hz, 0.1Hz and 0.2Hz; see (3) for details)), measured at 7T. We measured response amplitudes for each stimulus frequency in the parenchyma and in veins. We then compared our findings to HRF model predictions, to test the hypothesis that responses to fast stimuli attenuate more in veins than in the parenchyma.
Methods
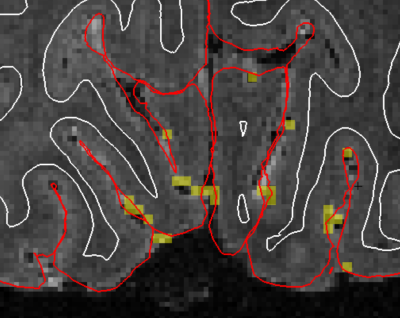
Data were acquired from 12 participants (three excluded). In each session one anatomical MEMPRAGE and ten BOLD fMRI scans (duration: 260s each, stimulus frequencies: 2 runs at 0.05Hz, 3 at 0.10Hz, 6 at 0.20Hz) were collected. BOLD dataset were acquired with 1.06mm isotropic resolution, GRAPPA 4, no PF, 174x174mm FOV, TR=0.874s, 16 slices positioned over the calcarine sulci, PE=L->R, TE=24ms, flip=52, ES=0.81ms. Functional data were slice-time and motion-corrected, and registered to the subject-specific functional localizer. Runs of the same frequency were averaged. Response time-series were interpolated to 100ms sampling, and oscillatory periods (trials) were averaged. Voxelwise estimates of oscillation amplitude were obtained by fitting a sinusoid and its first harmonic (to account for shape variability) to the trial-averaged response. Veins were identified as the top 0.3% of active voxels in the localizer run, and within the top 20% of the cortical gray matter. The 0.3% threshold was estimated by overlaying the activation map on a T2* weighted 0.3mm isotropic gradient-echo (GRE) scan obtained on a separate session for subject 1, where vessels were readily identifiable (Fig. 2). Estimates of cortical depth were obtained with FreeSurfer. All analyses were constrained to voxels within the calcarine sulcus.Results
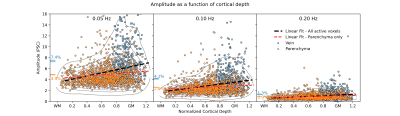
We first investigated how responses to fast stimuli varied across compartments. Consistent with prior studies, veins showed larger and slower responses than the parenchyma (Fig. 3), and response amplitudes declined for faster stimuli.Next, we looked at how voxelwise response amplitudes changed as a function of cortical depth across stimuli (Fig. 4). For slow 0.05 Hz stimulation (left), we found that veins represented a distinct, sparse subset of responses. Parenchymal responses were largely consistent across depths (slope in parenchyma at 0.05 Hz (95% conf.): 1.08 +- 0.59), suggesting that, especially at low frequencies, commonly observed depth-dependent differences in signal amplitudes may be largely due to sparse veins (slope in all voxels at 0.05 Hz (95% conf.): 2.90 +- 0.57), in agreement with earlier findings (4,5). For fast stimuli (Fig. 4, 0.20Hz), the venous responses remained larger than parenchymal responses, although their absolute differences were reduced.
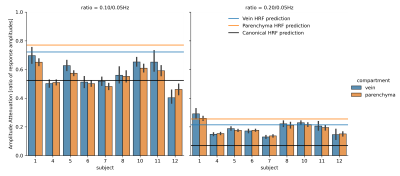
To test whether these compartments showed distinct stimulus-dependent effects, we computed the ratio of amplitudes for each voxel across stimuli, which we call amplitude attenuation. This ratio was computed for pairs: 0.05Hz vs. 0.10Hz, and 0.05Hz vs. 0.20Hz. Lower values indicate more attenuation with faster stimuli. We compared values to the canonical Glover (6) and fast Siero HRFs. Fig. 5 shows that at low frequencies (0.05 and 0.10 Hz), the measured attenuation ratios were closer to the canonical HRF predictions (black line). At the faster (0.20 Hz) frequency, however, the measured attenuation was less pronounced than the canonical HRF would have predicted - by at least a factor of two, and closer to fast HRF (2) predictions. We next investigated whether these fast responses differed between veins and parenchyma, but found no significant difference in attenuation between compartments (p>0.05).
Discussion
We found that when using fast stimuli, the measured responses in both veins and parenchyma are larger than canonical models predict. But why do veins not attenuate faster than the parenchyma? The attenuation for the 0.10Hz stimulation more closely matched the canonical HRF, whereas for 0.20Hz it more closely matched predictions from faster HRFs. This suggests the HRF itself may be accelerating as a function of stimulus frequency (7,8), causing both compartments to produce strong responses to fast stimuli. Overall, our results demonstrate that fast fMRI signals can be detected both in veins and in parenchyma, suggesting that both compartments may exhibit a nonlinear speeding of the HRF in response to fast stimuli. Future work should investigate these temporal properties at even faster timescales, where other dynamics such as phase cancellation (9) take effect, which could produce distinct consequences in veins and parenchyma.Acknowledgements
This work was supported by National Institutes of Health grants P41-EB015896, R21-NS106706, R01-EB019437, P41-EB030006, R00-MH111748, R01-AG070135, and by the MGH/HST Athinoula A. Martinos Center for Biomedical Imaging; and was made possible by the resources provided by NIH Shared Instrumentation Grants S10-RR019371.References
1. Menon RS. The Great Brain Versus Vein Debate. NeuroImage 2012;62:970–974 doi: 10.1016/j.neuroimage.2011.09.005.
2. Siero JC, Petridou N, Hoogduin H, Luijten PR, Ramsey NF. Cortical Depth-Dependent Temporal Dynamics of the Bold Response in the Human Brain. Journal of Cerebral Blood Flow & Metabolism 2011;31:1999–2008 doi: 10.1038/jcbfm.2011.57.
3. Lewis LD, Setsompop K, Rosen BR, Polimeni JR. Fast Fmri Can Detect Oscillatory Neural Activity in Humans. Proceedings of the National Academy of Sciences 2016;113:E6679–E6685 doi: 10.1073/pnas.1608117113.
4. Kay K, Jamison KW, Zhang R-Y, U\gurbil K. A Temporal Decomposition Method for Identifying Venous Effects in Task-Based Fmri. Nature Methods 2020;17:1033–1039 doi: 10.1038/s41592-020-0941-6.
5. Zhang N, Zhu X-H, Chen W. Investigating the Source of Bold Nonlinearity in Human Visual Cortex in Response To Paired Visual Stimuli. NeuroImage 2008;43:204–212 doi: 10.1016/j.neuroimage.2008.06.033.
6. Glover GH. Deconvolution of Impulse Response in Event-Related Bold Fmri1. NeuroImage 1999;9:416–429 doi: 10.1006/nimg.1998.0419.
7. Lewis LD, Setsompop K, Rosen BR, Polimeni JR. Stimulus-Dependent Hemodynamic Response Timing Across the Human Subcortical-Cortical Visual Pathway Identified Through High Spatiotemporal Resolution 7t Fmri. NeuroImage 2018;181:279–291 doi: 10.1016/j.neuroimage.2018.06.056.
8. Birn RM, Saad ZS, Bandettini PA. Spatial Heterogeneity of the Nonlinear Dynamics in the Fmri Bold Response. NeuroImage 2001;14:817–826 doi: 10.1006/nimg.2001.0873.
9. Chen JE, Glover GH, Fultz NE, Rosen BR, Polimeni JR, Lewis LD. Investigating Mechanisms of Fast Bold Responses: the Effects of Stimulus Intensity and of Spatial Heterogeneity of Hemodynamics. NeuroImage 2021;245:118658 doi: 10.1016/j.neuroimage.2021.118658.
Figures