2114
A novel free-running framework for Blood Oxygen Level Dependent functional MRI1Department of Diagnostic and Interventional Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 2CIBM Center for Biomedical Imaging, Lausanne, Switzerland, 3Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 4LTS5, Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 5Department of Psychology, Faculty of Psychology and Educational Sciences, University of Geneva, Geneva, Switzerland, 6Department of Hearing and Speech Sciences, Vanderbilt University, Nashville, TN, United States, 7These authors provided equal last-authorship contribution, Lausanne, Switzerland
Synopsis
We demonstrate the efficacy of a new free-running framework for quantitatively measuring blood-oxygen-level-dependent functional MRI (BOLD-fMRI) signals. Whole-brain data were acquired uninterruptedly during a blocked-design ON/OFF visual paradigm (checkerboard vs. grey image). 5-D volumes were reconstructed (i.e. the three spatial dimensions (x,y,z), the trial dimension (N=33), and the ON vs. OFF dimension (N=2)). BOLD-activated regions were measured as the difference between volumes reconstructed from all read-outs acquired during the ON vs. OFF condition, rather than resulting from a canonical, model-based, GLM analysis. The framework enables high temporal T2* k-space sampling and opens up new horizons for BOLD-fMRI.
Introduction
Blood-oxygen-level-dependent functional MRI (BOLD-fMRI) has been successfully used to map cognitive functions in the brain since its discovery in the 1990s1,2. Current brain fMRI pipelines correct for motion, which causes large artefacts in the BOLD signal. However, despite the application of motion correction, the statistical significance of BOLD activation obtained in the presence of motion remains much lower than no-motion conditions3. Another limitation is the achievable temporal resolution, since adequate signal quality for a whole-brain acquisition is typically obtained with repetition times TR on the order of 2s and even accelerated acquisitions being on the order of 400ms4. This temporal sampling impedes the ability to directly relate BOLD-fMRI to other physiological signals that unfurl at a faster rate than ~0.5Hz. Here we introduce a new free-running framework for quantitative BOLD-fMRI, where the percentage signal change attributed to neural activity arises as the contrast between two reconstructed volumes, obtained by binning together independent radial read-outs. The proposed framework aims to improve over existing BOLD-fMRI methods in that it is 1) flexible, as read-outs can be retrospectively and arbitrarily binned together to extract different information (i.e. T2*-weighted anatomical volumes or the hemodynamic response function-hrf), 2) motion-robust, as the use of compressed-sensing together with motion rejection strategies allows for high-resolution T2*-weighted motion-consistent volume reconstructions, 3) quantitative, as the extraction of the BOLD map does not depend on any statistical modelling at the subject level and 4) sampled at high temporal rate, as independent readouts are acquired with TR=28.24ms.Methods
Participants and visual stimulation. Twenty healthy volunteers were scanned in a 3T clinical scanner (MAGNETOM PrismaFit, Siemens Healthcare, Erlangen, Germany) with a 64-channel head coil. Visual stimuli were back-projected onto a mirror attached to the head coil. The study protocol was approved by the local ethics committee. The visual field on the mirror spanned 18°. The stimulation protocol followed a blocked design of 33 trials of 40s. One trial entailed a 15s ON phase (8Hz flickering checkerboard) followed by a 25s OFF phase (full-field grey patch). This sequence allows BOLD signal to reach its peak (after ~5s) and to return to baseline (after ~30s).Acquisition. Data were acquired using a prototype uninterrupted gradient recalled echo (GRE) sequence with a 3D radial spiral phyllotaxis sampling trajectory5 that enables uniform k-space coverage in all bins. The acquisition and the visual stimulation were synchronized via a Syncbox (NordicNeuroLab). A total of 46,772 readouts were acquired with TR=28.24ms, TE=25ms, FoV=192mm3 with 1mm3 isotropic resolution, and FA=12°, TA = 22 min. An high resolution anatomical T1 weighted volume (MPRAGE, TE= 2.43ms, TR=1890ms, FA=9°, FOV=256x256, 192 slices, voxel=1mm3 isotropic) was acquired as basis for ROIs segmentation.
Reconstruction. We performed a 5D (x-y-z-rep-act dimensions) image reconstruction6, where rep corresponds to the trials dimension (N=33 bins) and act to the ON vs. OFF dimension (N=2 bins) with a k-t-sparse SENSE algorithm7,8 (image undersampling 20%), resulting in 66 3D volumes. A second 4D (x-y-z-t dimensions, where t corresponds to 16 bins of 2.5s) image reconstruction was also performed. For the ON-phase, readouts acquired over the 5-15s trial time interval were retained for binning, and for the OFF-phase readouts acquired over the 30-40s trial time interval were retained (Figure 1b). All other readouts were discarded to mimic the hemodynamic response active vs. rest phases. Total variation regularization was applied along the rep dimension, as readouts acquired during the same hemodynamic phase but across different trials share information. Fourier transform regularisation was applied along the temporal dimension of the 4D reconstruction (Figure 1b). Motion correction translation and rotational coefficients were estimated using SPM129 and used to correct the original k-space. Regions of interest (ROIs) were extracted using FreeSurfer1 at subject-level and included the calcarine cortex (primary visual cortices), superior temporal sulci (primary auditory cortices) and the postcentral gyrus (somatosensory cortices).
Results
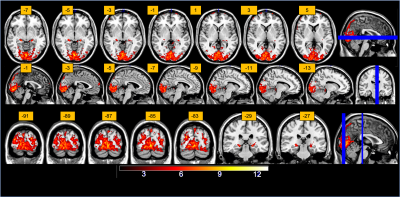
The percentage signal changes estimated from the 5D reconstruction in a set of ROIs during visual stimulation demonstrate the efficacy of the technique. As expected, we were able to retrieve a consistent percent signal change (1%) in the primary visual cortices (STD of the signal 0.43%), whereas all the other extracted ROIs showed no activation (<0.05%). The signal extracted from the alternative 4D reconstruction and binning (Figure 2) in these ROIs, confirmed the presence of canonical hemodynamic shaped response (Figure 2). The inference at a group level was computed as a paired-t-test (p<0.001, extended threshold of 100 contiguous voxels) and show canonical activation in the visual cortices as well as the lateral geniculate nuclei (Figure 3).Discussion
The proposed framework shows robustness and flexibility in reconstruction options compared to current BOLD-fMRI acquisition protocols based on EPI. The compressed-sensing reconstruction helps mitigating noise, while the radial high-sampling rate of k-space guarantees high flexibility in the way volumes can be reconstructed. From the same acquisition, we can retrieve either ON vs OFF volumes or time-resolved volumes spanning the whole trial duration; from the mathematical difference (abs ( || ON || - || OFF || ) we obtain BOLD contrast maps, equivalent to those estimated via a classical GLM analysis, but without the GLM-induced model assumptions. Conversely, the time-resolved reconstruction allows us to see the hrf signal evolution spanning the 40s stimulation duration.Acknowledgements
No acknowledgement found.References
1. Ogawa, S., Lee, T. M., Kay, A. R. & Tank, D. W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. U. S. A. 87, 9868–72 (1990).
2. Ogawa, S. et al. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc. Natl. Acad. Sci. 89, 5951–5955 (1992).
3. Zaitsev, M., Akin, B., LeVan, P. & Knowles, B. R. Prospective motion correction in functional MRI. Neuroimage 154, 33–42 (2017).
4. Narsude, M., Gallichan, D., van der Zwaag, W., Gruetter, R. & Marques, J. P. Three-dimensional echo planar imaging with controlled aliasing: A sequence for high temporal resolution functional MRI. Magn. Reson. Med. 75, 2350–2361 (2016).
5. Piccini, D., Littmann, A., Nielles-Vallespin, S. & Zenge, M. O. Spiral phyllotaxis: The natural way to construct a 3D radial trajectory in MRI. Magn. Reson. Med. 66, 1049–1056 (2011).
6. Lorenzo Di Sopra, Davide Piccini, Simone Coppo, Matthias Stuber, J. Y. An automated approach to fully self‐gated free‐running cardiac and respiratory motion‐resolved 5D whole‐heart MRI. Magn. Reson. Med. (2019).
7. Lustig, M., Donoho, D. L., Santos, J. M. & Pauly, J. M. Compressed Sensing MRI. IEEE Signal Process. Mag. 25, 72–82 (2008).
8. Lustig, M., Donoho, D. & Pauly, J. M. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn. Reson. Med. 58, 1182–1195 (2007).
9. Andersson, J. et al. Statistical Parametric Mapping: The Analysis of Functional Brain Images. (ACADEMIC PRESS, 2006).
10. Fischl, B. FreeSurfer. Neuroimage 62, 774–781 (2012).
Figures
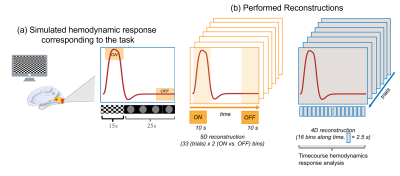
Figure 1 (a) Simulated hemodynamic response corresponding to the task. (b) Performed reconstruction. In orange, the 5D reconstruction for BOLD maps extraction with resolved trials and ON-OFF response, in blue the 4D reconstruction with the resolved hemodynamic responses.
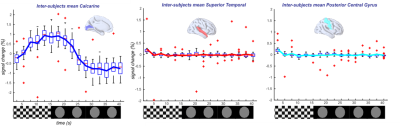
Figure 2. (Left) Measured inter-subject percental signal change in the visual areas. (Center) Average signal below 0.05% in temporal regions. (Right) Average signal below 0.05% in posterior central gyrus. Box-whisker plots show medians, interquartile ranges, and 95%-confidence intervals; outliers are displayed in red.