2113
Combining the benefits of 3D acquisitions and spiral readouts in VASO fMRI1Maastricht University, Maastricht, Netherlands, 2CEA NeuroSpin, Gif-sur-Yvette, France
Synopsis
VASO fMRI can provide beneficial localization specificity and quantifiability compared to the commonly used BOLD contrast. Previous work has also shown the benefits of using spiral readouts compared to Cartesian.
In this work, we explore the benefits of 3D stack of spirals readouts and compare it with the current state of the art 3D EPI readouts for VASO fMRI. The sequence implementation is done using Pulseq, images were reconstructed using gpuNUFFT; functional analysis with an openly available pipeline. We find that a tSNR efficiency improvement of a factor of 2.5 over EPI is achieved using the proposed spiral implementation.
Introduction
It has previously been shown that fMRI methods that measure cerebral blood volume. As such, VAscular Occupancy (VASO) [1] can outperform the most commonly used BOLD techniques with respect to its physiological interpretability and its localization specificity; this is especially the case at ultra-high fields and at a high spatial resolution (<1.5 mm) [2]. Some of the main limitations of VASO are BOLD contamination (which affects the VASO contrast), limited detection sensitivity, and temporal sampling efficiency. For the BOLD contamination, it has been shown that a BOLD-corrected VASO image can be obtained by means of dynamic division of concomitantly acquired control images [3]. To overcome the limitations set by low temporal sampling efficiency, previous work has suggested combining the efficiency of spiral k-space sampling with simultaneous multi-slice acquisitions [4]. For high-resolution fMRI, however, 3D readouts can be advantageous over 2D readouts [2].The purpose of this study is to combine the benefits of 3D acquisitions and the efficiency of spiral readouts to obtain VASO images at sub-second TR. This is possible using a 3D stack of spirals (SOSP) readout which has previously been advocated as an efficient framework for BOLD fMRI [5]. Here, we demonstrate that fMRI with VASO contrast can be obtained using SOSPs, allowing for shorter echo times to improve temporal SNR, remove BOLD contamination and reduce acquisition time.
Methods
A 3D stack of spirals SS-SI VASO method was implemented using Pulseq [6], a framework that allows for rapid prototyping and implementation of sequences in the scanner. For BOLD contamination correction, a BOLD weighted control image was acquired right after the VASO one [2]. The VASO specific inversion was implemented by means of a 10 ms TR-FOCI pulse [7] applied 900 ms before the first excitation pulse of the spiral-out readout module. The sequence diagram used in this work is shown in Figure 1.To confirm the stability of the novel sequence setup, a healthy volunteer was scanned using a 7T Siemens scanner, SIEMENS Healthineers while performing a motor task (block-design). Two different scenarios were tested: 1) low-resolution with parameters of 1.4x1.4x1 mm3, 24 slices, TRvol=767 ms, TE=2.5 ms, TI1=1283 ms with in-plane acceleration of 2.2 and 2) high-resolution: 0.9x0.9x1.0 mm3, 24 slices, TRvol=1381 ms, TE=2.5 ms, TI1=1590 ms with in-plane acceleration of 2.2. As a reference, a Cartesian 3D EPI [8] VASO was acquired with the same task and spatial resolution. The desired voxel size, coverage, inversion delay and acceleration was matched to the protocols of SOSPs. In order to (partly) account for the lower sampling efficiency of the Cartesian readout compared to spirals, partial Fourier imaging of 6/8 was used. The TE and TR of the Cartesian readout were kept as short as possible with TRvol/TE=2319/20 ms (for low resolutions) and TRvol/TE=3400/36 ms (for high resolutions), respectively. The image reconstruction was performed using the open-source software gpuNUFFT [9] and a CG Sense reconstruction. For functional analysis, the openly available VASO pipeline [https://github.com/layerfMRI] consisting of Motion correction, BOLD correction and conventional quality measures (tSNR, mean, GLM statistics) was used to analyze both the SOPs and 3D EPI acquisitions.
Results and Discussion
Figure 2 shows various quality metrics of the low-resolution SOSPs data. Figures 3 and 4 show the activation maps obtained from the low and high-resolution acquisitions respectively. Figure 5 shows that for both low and high-resolution acquisitions a higher tSNR efficiency can be obtained with the proposed implementation. Especially in the thermal noise dominated regime of the high-resolution VASO, the spiral approach outperforms the Cartesian sampling by a factor of 2.5. This is expected, as from cumulative gains of improved signal sampling, including shorter echo times with reduced T2*-decay and faster sampling with more images per unit of time. Without full integration of off-resonance correction in the spiral reconstruction scheme yet, the signal appears blurrier in the SOSPs compared to the Cartesian 3D EPI. Note however that this form of blurring refers to the signal only, not the noise. Thus, we do not expect that the tSNR estimates presented here are affected by this.Summary and Conclusion
In this work, we have shown that a fast implementation of a VASO fMRI with a 3D stack of spirals readout can be achieved using Pulseq. The preliminary results indicate that with the current implementation activation maps can be obtained at sub-second TR with considerable high tSNR. The application of off-resonance correction will further improve image quality. Even though spiral sampling allows for flexible acquisitions of fMRI data, the reconstruction is more challenging compared to conventional Cartesian sampling. Further work will focus on correcting for the B0 field inhomogeneities, optimizing the spiral trajectory, implementing acceleration in the kz direction, and reconstruction speed up. With this, we expect to be able to observe all the benefits of combining the efficiency of spirals and 3D acquisitions applied to VASO fMRI. We believe that the developed acquisition and analysis tools will be useful tools for future mesoscopic fMRI experiments at UHF, including the human 9.4T and 11.7T scanners available to this project.Acknowledgements
This work has been funded by the H2020 FET-Open AROMA grant agreement no. 88587.
Benedikt Poser is also funded by the NWO VIDI grant 16.Vidi.178.052, the National Institute for Health grant R01MH/111444 (PI David Feinberg).
Renzo Huber was funded from the NWO VENI project 016.Veni.198.032.
References
- Lu, H., Golay, X., Pekar, J. J., & Van Zijl, P. C. (2003). "Functional magnetic resonance imaging based on changes in vascular space occupancy". Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 50(2), 263-274.
- Huber, L., Ivanov, D., Handwerker, D. A., Marrett, S., Guidi, M., Uludağ, K., … Poser, B. A. (2018). “Techniques for blood volume fMRI with VASO: From low-resolution mapping towards sub-millimeter layer-dependent applications.” NeuroImage, 164, 131–143.
- Huber, Laurentius et al. (2014). “Slab-Selective, BOLD-Corrected VASO at 7 Tesla Provides Measures of Cerebral Blood Volume Reactivity with High Signal-to-Noise Ratio.” Magnetic Resonance in Medicine 72(1): 137–48.
- Zahneisen, B., Poser, B. A., Ernst, T., & Stenger, A. V. (2014). "Simultaneous Multi-Slice fMRI using spiral trajectories". NeuroImage, 92, 8-18.
- Hu, Y., & Glover, G. H. (2007). "Three‐dimensional spiral technique for high‐resolution functional MRI". Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 58(5), 947-951.
- Layton, K. J., Kroboth, S., Jia, F., Littin, S., Yu, H., Leupold, J., ... & Zaitsev, M. (2017). "Pulseq: a rapid and hardware‐independent pulse sequence prototyping framework". Magnetic resonance in medicine, 77(4), 1544-1552.
- Hurley, A. C., Al‐Radaideh, A., Bai, L., Aickelin, U., Coxon, R., Glover, P., & Gowland, P. A. (2010). "Tailored RF pulse for magnetization inversion at ultrahigh field". Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 63(1), 51-58.
- Poser, B. A., Koopmans, P. J., Witzel, T., Wald, L. L., & Barth, M. (2010). "Three dimensional echo-planar imaging at 7 Tesla". Neuroimage, 51(1), 261-266.
- Knoll, F., Schwarzl, A., Diwoky, C., & Sodickson, D. K. (2014). "gpuNUFFT-an open-source GPU library for 3D regridding with direct Matlab interface". In International Society for Magnetic Resonance in Medicine: Scientific Meeting & Exhibition (pp. 4297-4297).
Figures
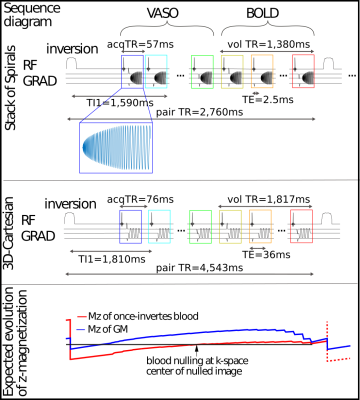
Figure 1: Sequence diagram of high-resolution acquisitions:
Upper: sequence diagram of high-resolution SOSP acquisition. A TR-FOCI inversion pulse is applied 900 ms before the excitation of the first volume. A BOLD weighted volume is obtained after the VASO one for further BOLD correction.
Middle: sequence diagram of high-resolution 3D Cartesian acquisition.
Lower: Expected evolution of z-magnetization of once inverted blood and Gray Matter. The k-space center is acquired at the blood nulling point.
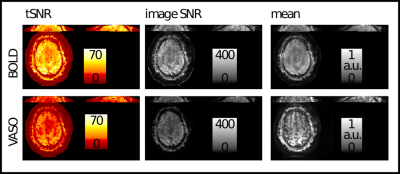
Figure 2: Basic image quality metrics of representative SOSP results: tSNR, image SNR and mean fMRI signal.
tSNR values (clearly above 30) suggest that even with relatively high spatial resolutions of 1.4x1.4x1mm3, the data are still not fully in the thermal noise dominated regime. The temporal mean image shows the characteristic T1 weighting of VASO, as expected. Note, that unlike conventional fMRI data, the short TEs of spiral acquisitions result in a relatively strong fat ring around the brain.
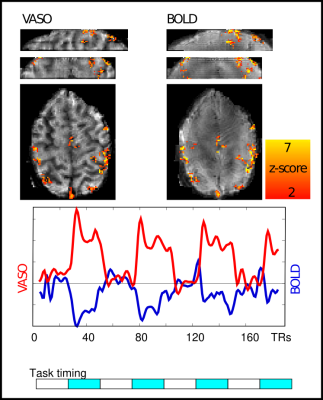
Figure 3: Activation maps, VASO and BOLD signal of low-resolution stack of spirals:
Temporal signal traces follow the task blocks as expected. Note that VASO is a negative CBV contrast with an expected signal decrease during activity. The representative results shown here highlight the high functional detection sensitivity of the implemented sampling approach. Three 30sec task blocks are sufficient to capture significant CBV changes in the cortex at 1.4x1.4x1mm3 resolution.
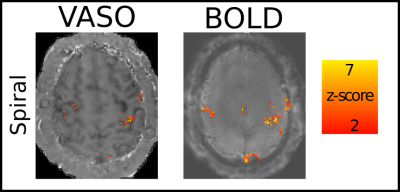
Figure 4: Activation maps of the high-resolution stack of spirals acquisition at 0.9x0.9x1.0 mm3.
Activation maps were also obtained with the high-resolution acquisition. It can be seen that the high sampling efficiency of the spiral readout provides sufficient detection sensitivity to capture functional CBV changes at sub-millimeter resolutions.
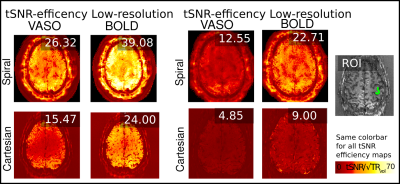
Figure 5: tSNR efficiency comparison spiral and Cartesian of low resolution and high-resolution data: spiral high-resolution VASO is about 2.5 times improved compared to Cartesian (12.55 compared to 4.85).
The values in the inset refer to the ROI of GM in the primary motor cortex (see ROI inset).