2108
Multi-level fiber tractography in brain tumor patients using functional mapping for seeding
Andrey Zhylka1, Josien Pluim1, Florian Kofler2,3,4, Ahmed Radwan5,6, Alberto De Luca7,8, Axel Schroeder9, Benedikt Wiestler2,4, Jan S. Kirschke2,10, Bjoern Menze3,11, Stefan Sunaert5,6,12, Alexander Leemans7, Sandro M. Krieg9,13, and Nico Sollmann2,13,14
1Biomedical Engineering, Eindhoven University of Technology, Eindhoven, Netherlands, 2Department of Diagnostic and Interventional Neuroradiology, School of Medicine, Klinikum rechts der Isar, Technical University of Munich, Munich, Germany, 3Department of Informatics, Technical University of Munich, Munich, Germany, 4TranslaTUM - Central Institute for Translational Cancer Research, Technical University of Munich, Munich, Germany, 5Department of Imaging and Pathology, Translational MRI, KU Leuven, Leuven, Belgium, 6Department of Neuroscience, Leuven Brain Institute (LBI), KU Leuven, Leuven, Belgium, 7Image Sciences Institute, University Medical Center Utrecht, Utrecht, Netherlands, 8Neurology Department, UMC Utrecht Brain Center, University Medical Center Utrecht, Utrecht, Netherlands, 9Department of Neurosurgery, School of Medicine, Klinikum rechts der Isar, Technical University of Munich, Munich, Germany, 10TUM-Neuroimaging Center, Klinikum rechts der Isar, Technical University of Munic, Munich, Germany, 11Department of Quantitative Biomedicine, University of Zurich, Zurich, Switzerland, 12Department of Radiology, UZ Leuven, Leuven, Belgium, 13TUM-Neuroimaging Center, Klinikum rechts der Isar, Technical University of Munich, Munich, Germany, 14Department of Diagnostic and Interventional Radiology, University Hospital Ulm, Ulm, Germany
1Biomedical Engineering, Eindhoven University of Technology, Eindhoven, Netherlands, 2Department of Diagnostic and Interventional Neuroradiology, School of Medicine, Klinikum rechts der Isar, Technical University of Munich, Munich, Germany, 3Department of Informatics, Technical University of Munich, Munich, Germany, 4TranslaTUM - Central Institute for Translational Cancer Research, Technical University of Munich, Munich, Germany, 5Department of Imaging and Pathology, Translational MRI, KU Leuven, Leuven, Belgium, 6Department of Neuroscience, Leuven Brain Institute (LBI), KU Leuven, Leuven, Belgium, 7Image Sciences Institute, University Medical Center Utrecht, Utrecht, Netherlands, 8Neurology Department, UMC Utrecht Brain Center, University Medical Center Utrecht, Utrecht, Netherlands, 9Department of Neurosurgery, School of Medicine, Klinikum rechts der Isar, Technical University of Munich, Munich, Germany, 10TUM-Neuroimaging Center, Klinikum rechts der Isar, Technical University of Munic, Munich, Germany, 11Department of Quantitative Biomedicine, University of Zurich, Zurich, Switzerland, 12Department of Radiology, UZ Leuven, Leuven, Belgium, 13TUM-Neuroimaging Center, Klinikum rechts der Isar, Technical University of Munich, Munich, Germany, 14Department of Diagnostic and Interventional Radiology, University Hospital Ulm, Ulm, Germany
Synopsis
Navigated transcranial magnetic stimulation (nTMS) motor mapping allows locating function in the individual patient pre-operatively and, to a certain extent, decreases the false-positive rate for fiber tractography. However, the false-negative rate is still high. In this work we evaluate novel multi-level fiber tractography (MLFT) along with conventional deterministic algorithms in patients with motor-eloquent high-grade glioma, combined with nTMS mapping-based region of interest (ROI) placement. The results were compared based on the coverage of nTMS motor maps, revealing reconstruction of the corticospinal tract (CST) with significantly higher volume and better coverage of the eloquent motor cortex for MLFT.
Introduction
Despite diffusion MRI (dMRI) offers an in-vivo insight into neuroanatomy by performing white-matter fiber tractography1. However, conventional approaches are known to have limitations and may produce false-positive and false-negative pathways2. Furthermore, placement of regions of interest (ROIs) for subsequent tractography can be challenging. This holds in particular for the common case of patients with functionally eloquent brain tumors, (due to altered anatomo-functional architecture and functional reallocation3,4,5). nTMS allows locating function in the individual patient pre-operatively and enables ROI definition based on the individual functional motor map6,7. Combined with fiber tractography, nTMS can provide valuable input for surgery planning in patients with motor-eloquent lesions6,7.However, the false-negative rate is still high in tractography. In this work we evaluate recently proposed MLFT8 along with deterministic diffusion tensor imaging (DTI)-based as well as constrained spherical deconvolution (CSD)-based tractography in patients with motor-eloquent high-grade glioma. Pre-operative nTMS motor mapping was used for ROI definition. We evaluated the aforementioned algorithms based on their coverage of the nTMS motor maps and explored the effect of an angular-deviation threshold (ADT) as well as fractional anisotropy threshold (FAT).
Methods
We used pre-operative data of 31 adult patients diagnosed with high-grade glioma in motor-eloquent location. All patients underwent functional mapping of upper and lower extremity motor function using nTMS (Nexstim eXimia NBS system, Nexstim Plc.), providing an individual motor map based on motor-evoked potentials elicited by neuronavigated single-pulse stimulation6,7. 3-Tesla dMRI (Achieve dStream, Philips Healthcare) was acquired with one volume at b=0s/mm2 and 32 volumes at b=1000s/mm2 with uniformly distributed gradient directions and a voxel size of 2 mm isotropic. Brain parcellation was performed using FreeSurfer9, preceded by lesion segmentation and lesion filling10,11,12. The dMRI data were corrected for motion and eddy currents, and co-registered to the corresponding T1-weighted images using ExploreDTI13. The fiber orientation distributions (FODs) were estimated using recursive calibration of the response function14. A spherical harmonics order of $$$L_{max}=6$$$ was used15.We performed tractography using MLFT, deterministic CSD-based, and deterministic DTI-based algorithms. Three ADTs (20°/45°/60°) and a FOD peak threshold of 0.08 (for MLFT and CSD-based tractography) was used. For DTI-based tractography, three FATs (25%/50%/75% FAT) were considered, referring to the minimum threshold enabling reconstructing a minimum fiber course (i.e., 100% FAT)16. The seed region was defined as a cross-section at the anterior pontine level of the brain stem (Figure 1)17 . The target region for tractography was obtained by enlarging each motor-positive nTMS point by a hull of 2 mm radius (Figure 1)6,7,18. The ratio of connected spots was counted per each algorithm and parameter configuration, providing the coverage of the nTMS motor map, where connected spots are motor-positive nTMS points (with 2-mm hulls) visited by at least one pathway of a reconstructed bundle. The significance of the parameter changes was estimated using Wilcoxon tests. In addition, we calculated the volumes of the reconstructed CST.
Results
Compared to DTI- and CSD-based algorithms, MLFT was capable of reconstructing the CST with higher volumes than the DTI- and CSD-based algorithms, (Figure 1, Table 1). Moreover, coverage of the nTMS motor map (i.e., motor-positive nTMS spots with their hull that are visited by reconstructed fibers) was highest for MLFT, with statistically significant differences compared with DTI- and CSD-based algorithms (in all comparisons using Wilcoxon tests with alternative hypothesis that MLFT-produced coverage is lower: p<0.01 each; Figure 2, Table 3).The CSD-based algorithm showed performance comparable to the DTI-based algorithm at the lowest ADT, while the nTMS motor map coverage achieved with DTI-based tractography primarily varied with the change in FAT, (Table 2). Additionally, the change in ADT showed to be statistically significant in almost all instances (except for 75% FAT for DTI-based reconstruction; Table 3).
Discussion & Conclusion
The newly proposed MLFT provides reconstruction of the CST in patients with motor-eloquent high-grade glioma with larger bundle volume and coverage of the nTMS motor map than deterministic DTI- and CSD-based tractography. It was also shown to consistently gain the coverage by increasing the ADTs (Figure 2), similar to CSD-based tractography. DTI-based tractography, however, did not show practically significant changes with ADT alteration (Figure 2), despite the results at different ADTs being statistically significant in most cases, which may be an illustration of the model’s inability to resolve complex fiber configurations19. Given that DTI is widely applied in the distinct pre-operative setup of patients with functionally eloquent tumors but has well-known shortcomings20,21, MLFT may provide a more detailed and complete reconstruction of the CST compared to DTI-based fiber tractography.Furthermore, the results show the importance of the parameter setting choice for clinical application, (Figure 2, Table 3). Given that MLFT is capable of estimating high-angular fiber distributions, changing ADTs appears to be promising and should be tested and verified by intraoperative direct electrical stimulation as the next step. In case of DTI, experiments with FAT appear to be more beneficial for the improvement of the extent of reconstruction. In conclusion, MLFT could improve fiber tracking of the CST in patients with brain tumors beyond DTI, but attention has to be paid to the distinct parameter settings to obtain optimal results.
Acknowledgements
Andrey Zhylka is supported by the funding from the European Union's Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement No 765148.References
1. Jeurissen B., Descoteaux M., Mori S., Leemans A. Diffusion MRI tractography of the brain. NMR Biomed, 32(4):e3785 (2019)2. Maier-Hein KH, Neher PF, Houde JC, Cote MA, Garyfallidis E, Zhong J, et al. The challenge of mapping the human connectome based on diffusion tractography. Nat Commun 8, 1349 (2017)
3. Gibb W.R., Kong N.W., Tate M.C. Direct Evidence of Plasticity within Human Primary Motor and Somatosensory Cortices of Patients with Glioblastoma. Neural Plast 2020:8893708 (2020)
4. Bulubas L., Sardesh N., Traut T., Findlay A., Mizuiri D., Honma S.M., Krieg S.M., Berger M.S., Nagarajan S.S., Tarapore P.E. Motor Cortical Network Plasticity in Patients with Recurrent Brain Tumors. Front Hum Neurosc, 14:118 (2020)
5. Conway N., Wildschuetz N., Moser T., Bulubas L., Sollmann N., Tanigawa N., Meyer B., Krieg S.M. Cortical plasticity of motor-eloquent areas measured by navigated transcranial magnetic stimulation in patients with glioma. J of Neurosurgery, 127(5):981-991 (2017)
6. Sollmann N, Krieg SM, Säisänen L, Julkunen P. Mapping of Motor Function with Neuronavigated Transcranial Magnetic Stimulation: A Review on Clinical Application in Brain Tumors and Methods for Ensuring Feasible Accuracy. Brain Sci, 11(7):897 (2021)
7. Sollmann N, Meyer B., Krieg S. Implementing Functional Preoperative Mapping in the Clinical Routine of a Neurosurgical Department: Technical Note. World Neurosurg, 103:94-105 (2017)
8. Zhylka A., Leemans A., Pluim J., De Luca A. Anatomically informed multi-level fiber tractography for improved sensitivity of white matter bundle reconstruction in diffusion MRI. ISMRM (2020), p0855
9. Fischl B. FreeSurfer. NeuroImage, 62(2):774-81 (2012)
10. Radwan AM, Emsell L, Blommaert J, Zhylka A, Kovacs S, Theys T, et al. Virtual brain grafting: Enabling whole brain parcellation in the presence of large lesions. NeuroImage, 229:117731 (2021)
11. Kofler F, Berger C, Waldmannstetter D, Lipkova J, Ezhov I, Tetteh G, et al. BraTS Toolkit: Translating BraTS Brain Tumor Segmentation Algorithms Into Clinical and Scientific Practice. Front Neurosci, 14:125 (2020)
12. Menze BH, Jakab A, Bauer S, Kalpathy-Cramer J, Farahani K, Kirby J, et al. The Multimodal Brain Tumor Image Segmentation Benchmark (BRATS). IEEE Trans Med Imaging, 34(10):1993-2024 (2015)
13. Leemans, A., Jeurissen, B., Sijbers, J., Jones, D. K. ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. ISMRM; 3537 (2009).
14. Tax CM, Jeurissen B, Vos SB, Viergever MA, Leemans A. Recursive calibration of the fiber response function for spherical deconvolution of diffusion MRI data. NeuroImage, 86:67-80 (2014)
15. Tournier JD, Calamante F, Gadian DG, Connelly A. Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. NeuroImage, 23(3):1176-85 (2004)
16. Frey D, Strack V, Wiener E, Jussen D, Vajkoczy P, Picht T. A new approach for corticospinal tract reconstruction based on navigated transcranial stimulation and standardized fractional anisotropy values. NeuroImage, 62(3):1600-9 (2012)
17. Weiss C, Tursunova I, Neuschmelting V, Lockau H, Nettekoven C, Oros-Peusquens AM, et al. Improved nTMS- and DTI-derived CST tractography through anatomical ROI seeding on anterior pontine level compared to internal capsule. NeuroImage Clinical, 7:424-37 (2015)
18. Sollmann N, Wildschuetz N, Kelm A, Conway N, Moser T, Bulubas L, et al. Associations between clinical outcome and navigated transcranial magnetic stimulation characteristics in patients with motor-eloquent brain lesions: a combined navigated transcranial magnetic stimulation-diffusion tensor imaging fiber tracking approach. J of Neurosurgery, 128(3):800-810 (2018)
19. Farquharson S., Tournier J.-D., Calamante F., Fabinyi G., Schneider-Kolsky M., Jackson G. D., Connelly A. White matter fiber tractography: why we need to move beyond DTI. J of Neurosurgery 118(6): 1367-1377 (2013)
20. Duffau H. Diffusion tensor imaging is a research and educational tool, but not yet a clinical tool. World Neurosurg, 82(1-2):e43-5 (2014)
21. Nimsky C. Fiber tracking--we should move beyond diffusion tensor imaging. World Neurosurg, 82(1-2):35-6 (2014)
Figures
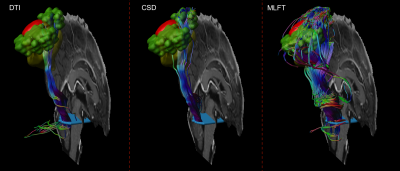
Figure 1. Exemplary case of nTMS-based
reconstruction of the CST. A cross-section of the brain stem (light blue)
placed at the anterior pontine level was used as a seed region for tractography.
The nTMS-based motor map (green; single motor-positive nTMS points with a 2-mm
hull) was used as the target region for reconstruction in the vicinity of a
tumor (red).
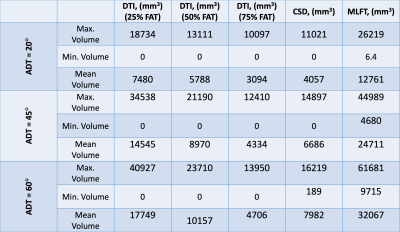
Table 1. Volumes
of the bundles reaching the nTMS motor map. For DTI three angular thresholds as
well as three FA thresholds are included.
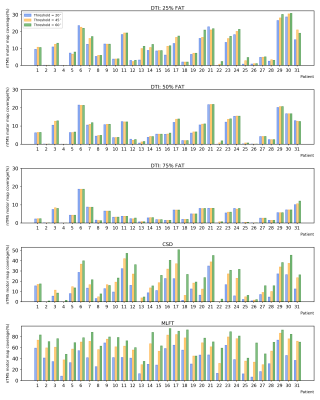
Figure
2. Ratio
of the nTMS motor maps that is visited by fiber bundles reconstructed with
DTI-based (with 25, 50, and 75% of the individual fractional anisotropy
threshold [FAT]), CSD-based, and MLFT tractography. Change of the
angular-deviation thresholds (ADTs) appears to only have visible effect on the
result of CSD-based tractography and MLFT.
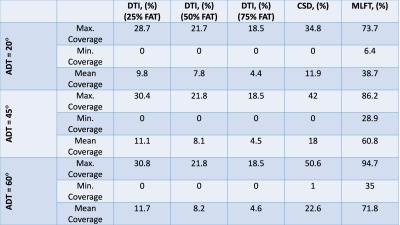
Table 2. The
nTMS motor map coverage by the three algorithms given varied angular threshold
as well as varied FA threshold in case of DTI.
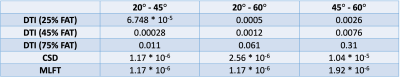
Table 3. P-values obtained from comparing the nTMS motor map
coverage given varied angular deviation thresholds (ADTs) using paired Wilcoxon
tests. Using a significance level α = 0.05, the difference caused by changing the ADT is
significant for all cases except at 75% of the individual fractional anisotropy
threshold (FAT) for DTI-based tractography.
DOI: https://doi.org/10.58530/2022/2108