2107
Investigation of the singularity of the chimpanzee brain superficial white matter bundles using diffusion MRI and clustering-based approaches1BAOBAB, NeuroSpin, Université Paris-Saclay, CNRS, CEA, Gif-sur-Yvette, France, 2Keeling Center for Comparative Medicine and Research, The University of Texas MD Anderson Cancer Center, Bastrop, TX, United States
Synopsis
A way to better appreciate the complex human brain evolution relies on comparative investigations with homologous species. We present here the superficial connectivity organization of the Chimpanzee brain using a combination of image processing and DBSCAN clustering analyzes obtained from 39 chimpanzees DTI scans. The results revealed the presence of U-fiber, V-fiber and Curved-fiber shapes already identified in Human superficial fiber connectivity studies. This non-negligeable short fibers organisation resemblance between Chimpanzees and Humans, brings out new perspectives on hominid brain singularity knowledge.
Introduction
Understanding the singularity of the primate brain is an exciting field of research aiming at better understanding the factors driving the high level cognitive abilities of these rare species that fascinate us. Recent advances in MRI have recently made it possible to map the superficial connectivity of the chimpanzee brain in vivo1. The exploration of the superficial chimpanzee brain microstructure and particularly its superficial organization, brings out new perspectives to compare the Chimpanzee and Human brains.Methods
Chimpanzee cohort - A cohort of 39 chimpanzees including 23 females and 16 males aged from 9 to 35 years old stemming from the National Yerkes Primate Research Center (NYPRC, Atlanta) was studied in the frame of an imaging protocol approved by the Local Animal Welfare Committee.Imaging protocol - All individuals were scanned using a Siemens Tim Trio 3T MRI system with a birdcage coil with an imaging protocol including a 0.625mm isotropic MPRAGE T1-weighted scan and a series of 5 1.9mm isotropic diffusion MRI (dMRI) scans using a single-short 2D PGSE sequence (TE/TR=86ms/6s, RBW=1563Hz/pixel, matrix 128x128, FOV=243.2mm) at b=1000s/mm² along 60 diffusion directions.
Pre/Post-processing - After correcting for imaging artifacts2 and matching all individuals to the Juna.Chimp template3 using diffeomorphic registration4, dMRI were used to compute analytical Q-ball ODF maps5 (SH order 6, Laplace-Beltrami regularization factor of 0.006) from which a whole brain regularized deterministic tractography6 was performed (1 seed/voxel, forward step 0.4mm, aperture angle 30°) to obtain individual tractograms. A fiber clustering algorithm7 was then applied to the set of tractograms relying on a twofold strategy: 1) intra-subject hierarchical fiber clustering of connectograms relying on a hemisphere-based, length range-based, and brain parcels-based partition of fibers to obtain individual clusters represented by their centroid; 2) cross-subject clustering using a density-based spatial clustering of the individual centroid maps (DBSCAN)8 using a corrected pairwise maximum Euclidean distance to define an affinity between centroids. The parameters were optimized in order to get an optimal number of clusters, yielding a normalisation factor nf=26, a centroid affinity maximum distance of 18mm, an affinity maximum distance after exclusion of 4mm, and an affinity variance of 140625mm².
Atlas definition - Superficial clusters corresponding to the fiber length range 7mm-50mm were then selected using the Davi130 parcellation atlas3. Each superficial cluster connecting two parcels A and B was attributed a label following the syntax A_B_Id (Id corresponding to the cluster index in the set of all clusters connecting parcels A and B), thus yielding a novel superficial white matter bundle atlas of the chimpanzee brain.
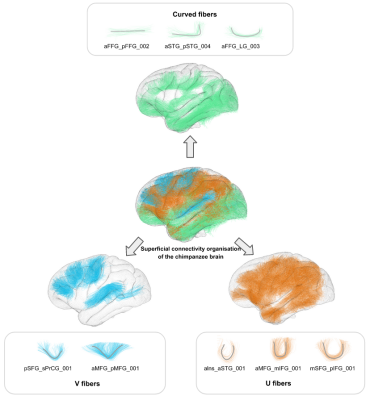
Cortical organisation - All the clusters were manually investigated based on shapes already found in the human short bundle atlas9, using defined inclusion criteria for each shape.
Results
39 individual whole brain tractograms were obtained, including a mean of 1503922+/-158933 fibers for each subject, from which an intra-subject fiber clustering step performed on short fibers provided 5400+/-747 clusters for the left hemisphere and 5473+/-737 clusters for the right hemisphere at the individual scale, being slightly higher for the left hemisphere. The resulting inter-subject fiber clustering yielded 4848 clusters aggregating a total amount of 240631/235746 fibers for the left and right hemispheres respectively. 112 pairs of regions from the left hemisphere were interconnected with a total of 422 clusters (aggregating 76845 fibers) whereas 114 pairs of regions from the right hemisphere were interconnected with a total of 404 clusters (aggregating to 74091 fibers)(see fig.2).Similar to humans9, the morphometry of superficial fiber clusters could be divided into 3 classes including ‘U’-shaped fibers, ‘V’-shaped fibers and ‘Curved’-shape fibers(fig.3). ‘U’-shaped fibers seem to support the different sulci, and are mostly found on the central part of the lateral face of the brain.‘V‘-shaped fibers are mainly found on the frontal lobe and make a bond between the lateral and the medial face of the brain, touching the frontal upper edge. ‘Curved’-shaped fibers are mainly found on the lower part of the brain, on the frontal and temporal lobe (except for fibers identified as subparts of the cingulum).
Discussion
To our knowledge, we present here the first structural organization of the short fiber bundles of the chimpanzee brain. While other atlases already exist concerning the Human brain7,9, the identification of such fiber shapes that are strikingly resembling the ones of Humans’ is a new step towards a better understanding of the commonalities and differences in the morphometry of the superficial connectivity of hominids. The limits of this study is the subjective point of view of the shape classification, implying the establishment of clear and parsimonious inclusion criteria, further to be confirmed with histological investigations.Acknowledgements
This work was partially funded by the Blaise Pascal Chair from Région Ile deFrance and the University Paris-Saclay to W. Hopkins.References
1. Chauvel M., Uszynski I., Hopkins W., Mangin J.-F., Poupon C. (2021- Monday, 17 May). A new superficial white matter connectivity atlas of the chimpanzee brain. The international society for magnetic resonance (ISMRM) conference, poster 1715. https://www.ismrm.org/21/program-files/TeaserSlides/TeasersSessions/94.html
2. Andersson, J. L., Skare, S., & Ashburner, J. (2003). How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage, 20(2), 870–888. https://doi.org/10.1016/S1053-8119(03)00336-7
3. Vickery, S., Hopkins, W. D., Sherwood, C. C., Schapiro, S. J., Latzman, R. D., Caspers, S., Gaser, C., Eickhoff, S. B., Dahnke, R., & Hoffstaedter, F. (2020). Chimpanzee brain morphometry utilizing standardized MRI preprocessing and macroanatomical annotations. eLife, 9, e60136. https://doi.org/10.7554/eLife.60136
4. Avants, B. B., Tustison, N. J., Song, G., Cook, P. A., Klein, A., & Gee, J. C. (2011). A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage, 54(3), 2033–2044. https://doi.org/10.1016/j.neuroimage.2010.09.025
5. Descoteaux, M., Angelino, E., Fitzgibbons, S., & Deriche, R. (2007). Regularized, fast, and robust analytical Q-ball imaging. Magnetic resonance in medicine, 58(3), 497–510. https://doi.org/10.1002/mrm.21277.
6. Perrin, M., Poupon, C., Cointepas, Y., Rieul, B., Golestani, N., Pallier, C., Rivière, D., Constantinesco, A., Le Bihan, D., & Mangin, J. F. (2005). Fiber Tracking in q-Ball Fields Using Regularized Particle Trajectories. Information processing in medical imaging : proceedings of the ... conference, 19, 52–63. https://doi.org/10.1007/11505730_5
7. Guevara, P., Duclap, D., Poupon, C., Marrakchi-Kacem, L., Fillard, P., Le Bihan, D., Leboyer, M., Houenou, J., & Mangin, J. F. (2012). Automatic fiber bundle segmentation in massive tractography datasets using a multi-subject bundle atlas. NeuroImage, 61(4), 1083–1099. https://doi.org/10.1016/j.neuroimage.2012.02.071
8. Ester, M., Kriegel, H., Sander, J., & Xu, X. (1996). A Density-Based Algorithm for Discovering Clusters in Large Spatial Databases with Noise. KDD. https://dl.acm.org/doi/10.5555/3001460.3001507
9. Labra N. (2020).
Inference of a U-fiber bundle atlas informed by the variability of the cortical folding pattern. Thesis. Université
Paris-Saclay. NNT : 2020UPAST056. http://www.theses.fr/2020UPAST056
Figures

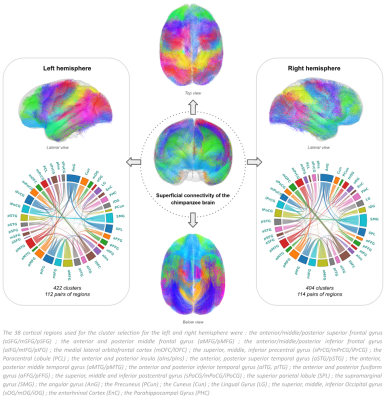
Figure 2. Superficial white matter connectivity atlas of the chimpanzee brain. 422 clusters, corresponding to 112 pairs of regions were extracted on the left hemisphere, and 404 clusters corresponding to 114 pairs of regions were extracted on the right hemisphere.