2084
Quantitative imaging of gene therapy delivery vehicles using CEST-NMR/MRI1Bioengineering, University of California, Berkeley, Berkeley, CA, United States
Synopsis
We hypothesized that AAV2 capsids may generate endogenous CEST contrast similar to LRP. We tested this using NMR CEST under varying pH, density, biological transduction stage, across serotypes and mixed biological media. Subsequent experiments determined the pH-dependent exchange rate and optimized CEST saturation schemes for AAV contrast detection at 7T. The results of this study reveal that AAV capsids generate endogenous CEST contrast around 0.6-0.8ppm. Exchangeable protons are likely from serine and threonine residues on the AAV capsid. Based on calculated fast exchange rates, an optimized CEST saturation scheme generated robust CEST contrast in mixed biological media phantoms at 7T.
Introduction
Over the last decade the field of gene therapy has rapidly expanded to include in vivo somatic cell gene editing1. Increasingly, adeno-associated virus (AAV) vectors have been exploited as delivery vehicles for gene editing machinery such as CRISPR thanks to non-pathogenicity, organ specificity, replication defectiveness, and low immunogenicity2. However, the reliance upon invasive biopsies to verify gene editing is a boundary to successful translation. Chemical exchange saturation transfer (CEST)-MRI has been used to image the expression of genetically encoded reporter genes including the Lysine Rich Protein (LRP)3-5. In comparison to the 50-residue LRP, AAV2 capsids contain over 1,000 surface Lysine residues6 that can potentially generate CEST contrast. We hypothesized that AAV2 capsids may generate endogenous CEST contrast similar to LRP. We tested this using NMR CEST under varying pH, density, biological transduction stage, and later across multiple serotypes and mixed biological media. Subsequent experiments determined the pH-dependent exchange rate and optimized CEST saturation schemes for AAV contrast detection at 7T.Methods
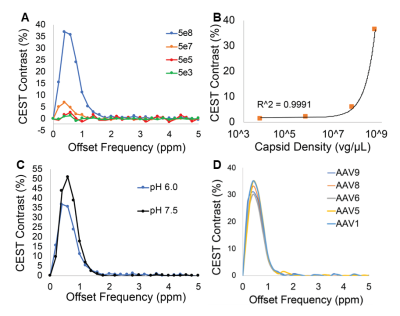
AAV Contrast at 800MHzNMR-CEST experiments (Bruker 800MHz) were performed on AAV2 (5.23×108 viral genomes/μL) in solution at pH 7.0 and 37°C. Complete Z-spectra (−5ppm to +5ppm) across a range of saturation powers (3.0μT to 9.0μT) were serially acquired for all studies. CEST contrast was calculated as MTRasym=(S(+ω)-S(−ω))/S0. First, MTRasym was assessed on AAV2 samples with varying capsid densities (5×103 vg/μL to 5×108 vg/μL), followed by pH values ranging from 4-7.5. CEST-NMR was next performed on multiple AAV serotypes (1, 5, 6, 7, 9). All AAV were commercially available (Vector Biolabs). To test whether AAV2 generates CEST contrast during endosomal transport, HEK293T cells were transduced with AAV2 (MOI 10k for 2 million cells) via one hour exposure at 4°C. Cells were harvested at 0-, 30-, and 60-minutes following removal of viral media. Control cells were exposed to normal media for one hour at 4°C. Afterwards, endosomes were lysed, isolated, and buffered in solution for NMR-CEST experiments.
Exchange rate quantification
Four samples of AAV2 (5.26×108 vg/μL) were titrated to pH values of 5.78, 6.07, 6.22, and 6.93 prior to imaging at 37° C. NMR-CEST Z-spectra (−10ppm to +10ppm) were acquired at varying saturation powers (1, 1.5, 2, 2.5, and 3μT). Fitting with Bloch-McConnell equations was then used for determining exchange rates for each sample.
Optimization at 7T
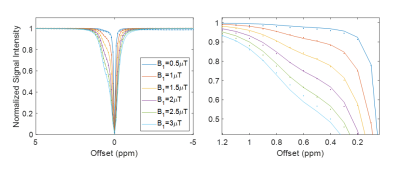
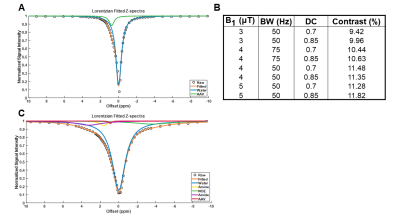
Phantoms containing AAV2 (5.26×108 vg/μL) were imaged at 7T (Bruker Pharmascan) with complete Z-spectra (−10ppm to +10ppm) acquired following varying values for saturation B1 (1, 1.5, 2, 3, 4 and 5μT), saturation pulse duration (54.8, 36.5, 27.4, 18.3, 13.7, 11ms), and saturation duty cycle (0.6, 0.7, 0.8, 0.85) across different combinations. CEST contrast was quantified by 2-pool Lorentzian fitting. In order to probe the impact of dilution in background tissue protein content, additional experiments were carried out on a phantom containing cell lysate (6.8mg protein) and AAV2 (5.26×108 vg/μL). Complete Z-spectra (−10ppm to +10ppm) were acquired following varying values for saturation B1 (3, 4 and 5μT), saturation pulse duration (54.8, 36.5, 27.4ms), and saturation duty cycle (0.7, 0.85) across all possible combinations. CEST contrast was quantified by 5-pool Lorentzian fitting with pools allocated for water, amine, amide, NOE, and AAV.
Results
Water-suppressed NMR of AAV2 identified 3 potential groups of high-density protons at 0.6, 3.0, and 3.6ppm relative to water (Figure 1A) of which only the exchangeable protons at 0.6ppm generated significant CEST contrast (Figure 1B) across a range of saturation powers (Figure 1C). CEST contrast of AAV2 at 0.6ppm correlated with capsid density and decreased with acidification (Figure 2). Similar CEST contrast was observed across other AAV serotypes (Figure 2D). CEST-NMR of isolated endosomes revealed a shift in resonant frequency to 0.8ppm, with contrast that peaked at 30 min after viral transduction (Figure 3). Quantification of viral titer and subsequent regression analysis revealed a positive linear correlation between CEST contrast and viral density (Figure 3). Z-spectra and subsequent fitting (Figure 4) at variable powers revealed an average exchange rate of approximately 1300Hz between pH 6-7. CEST contrast of AAV2 at 7T demonstrated a dependence upon saturation scheme parameters, but when optimized produced CEST contrast of 9-12% in phantoms (Figure 5A-B). Dilution of AAV2 capsids in cell lysate altered the Z-spectral appearance and reduced CEST contrast of AAV2 to approximately 3.3% (Figure 5C).Discussion
The results of this study reveal that AAV capsids generate endogenous CEST contrast around 0.6-0.8ppm. The most probable source of exchangeable protons is the conserved serine and threonine residues on the AAV capsid surface across serotypes. Based on the calculated exchange rates in the fast exchange regime, an optimized CEST saturation scheme generated robust CEST contrast in mixed biological media phantoms at 7T. Further optimization for in vivo imaging is ongoing.Acknowledgements
This study was supported by NIH 1R01HL28592-01, Silvian Foundation Award, AHA 19TPA34850040, NIH UH2EB028908-02, DGE 1752814References
1. Saha K, Sontheimer EJ, Brooks PJ, Dwinell MR, Gersbach CA, Liu DR, Murray SA, Tsai SQ, Wilson RC, Anderson DG, Asokan A, Banfield JF, Bankiewicz KS, Bao G, Bulte JWM, Bursac N, Campbell JM, Carlson DF, Chaikof EL, Chen ZY, Cheng RH, Clark KJ, Curiel DT, Dahlman JE, Deverman BE, Dickinson ME, Doudna JA, Ekker SC, Emborg ME, Feng G, Freedman BS, Gamm DM, Gao G, Ghiran IC, Glazer PM, Gong S, Heaney JD, Hennebold JD, Hinson JT, Khvorova A, Kiani S, Lagor WR, Lam KS, Leong KW, Levine JE, Lewis JA, Lutz CM, Ly DH, Maragh S, McCray PB Jr, McDevitt TC, Mirochnitchenko O, Morizane R, Murthy N, Prather RS, Ronald JA, Roy S, Roy S, Sabbisetti V, Saltzman WM, Santangelo PJ, Segal DJ, Shimoyama M, Skala MC, Tarantal AF, Tilton JC, Truskey GA, Vandsburger M, Watts JK, Wells KD, Wolfe SA, Xu Q, Xue W, Yi G, Zhou J; SCGE Consortium. The NIH Somatic Cell Genome Editing program. Nature. 2021;592(7853):195-204.
2. Kotterman MA, Chalberg TW, Schaffer DV. Viral Vectors for Gene Therapy: Translational and Clinical Outlook. Annu Rev Biomed Eng. 2015;17:63-89.
3. Gilad AA, McMahon MT, Walczak P, Winnard PT Jr, Raman V, van Laarhoven HW, Skoglund CM, Bulte JW, van Zijl PC. Artificial reporter gene providing MRI contrast based on proton exchange. Nat Biotechnol. 2007;25(2):217-9.
4. Farrar CT, Buhrman JS, Liu G, Kleijn A, Lamfers ML, McMahon MT, Gilad AA, Fulci G. Establishing the Lysine-rich Protein CEST Reporter Gene as a CEST MR Imaging Detector for Oncolytic Virotherapy. Radiology. 2015;275(3):746-54.
5. Meier S, Gilad AA, Brandon JA, Qian C, Gao E, Abisambra JF, Vandsburger M. Non-invasive detection of adeno-associated viral gene transfer using a genetically encoded CEST-MRI reporter gene in the murine heart. Sci Rep. 2018;8(1):4638.
6. Xie Q, Bu W, Bhatia S, Hare J, Somasundaram T, Azzi A, Chapman MS. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proc Natl Acad Sci U S A. 2002;99(16):10405-10.
Figures