2082
Optimization of pH-Weighted Contrasts in the Spinal Cord using Chemical Exchange Saturation Transfer (CEST) MRI1Medical Biophysics, Western University, London, ON, Canada, 2Centre for Functional and Metabolic Mapping, Robarts Research Institute, London, ON, Canada, 3Siemens Healthcare GmbH, Erlangen, Germany, 4Clinical Neurological Sciences, University Hospital, London Health Sciences Centre, London, ON, Canada
Synopsis
Degenerative cervical myelopathy (DCM) is a degenerative disease of the spine that leads to compression and neurological dysfunction. Recovery after surgery can be impacted by hypoxia in the cord, however the magnitude of this effect is currently unknown. Chemical Exchange Saturation Transfer (CEST) can produce contrast related to tissue pH, an indicator of hypoxia, but the method works best at ultra-high fields. Performing CEST in the spinal cord is also complicated by respiratory and cardiac motion and cerebrospinal fluid flow. The purpose of this work was to optimize pH-weighted CEST imaging in the human spinal cord at 3 Tesla.
Introduction
Degenerative cervical myelopathy (DCM) is a degenerative disease of the spine that causes compression of the spinal cord, leading to neurological dysfunction.1 Ischemia and hypoxia in the cord, caused by this compression, could impact recovery after decompression surgery. Unfortunately, direct in-vivo evidence of hypoxia and ischemia has been limited in humans. Chemical Exchange Saturation Transfer (CEST) is an MRI contrast derived from the transfer of magnetic saturation from selectively excited endogenous exchangeable protons, like amide and amine protons, to bulk water protons,2 causing a signal reduction in the observed water signal.3 The rate of exchange is pH dependent, and pH is altered by hypoxia. We have previously shown that pH-weighted CEST contrast can be generated in the brain using a ratiometric method called amine/amide concentration-independent detection (AACID).4 The use of low power radiofrequency (RF) pulses to saturate protons up field from water also produces contrast attributed to nuclear Overhauser enhancement (NOE),5,6 which originates from mobile macromolecules. Previous studies found that changes in tissue pH may cause the NOE effect to be lower in tumours, but this relationship is unclear and requires further study.5,7,8 Both AACID and NOE CEST contrast in the spinal cord could provide a means to examine pH change caused by hypoxia. Therefore, the objective of this study was to optimize AACID and NOE CEST contrast in the human spinal cord, creating sufficient sensitivity and resolution to detect pH heterogeneity in DCM patients.Methods
On a 3T Siemens MAGNETOM Prisma Fit MRI scanner, a prototype CEST sequence was used that incorporates a single slice 2D gradient echo (GRE) readout. Scan parameters that maximized AACID and NOE CEST effects were evaluated in egg white phantoms with differing pH. Pearson’s correlation coefficient (r) was used to assess the linear dependence of AACID and NOE effects on pH. Scan parameters were also varied in human brain (N = 3) CEST images to optimize in-vivo CEST contrast. Saturation was performed using Gaussian shaped radiofrequency (RF) pulses (pulse train length = 30, pulse length = 100 ms, interpulse delay = 1 ms) applied at 132 offsets from -6.5 to 6.5 ppm. The RF pulse amplitude was varied to find the optimal B1 value for AACID and NOE contrasts. Other relevant parameters include: TR/TE = 10.0/4.4 ms, voxel size = 2 mm x 2 mm, slice thickness = 5 mm and 1 average. To correct for B0 inhomogeneities, the CEST spectrum was shifted on a pixel-by-pixel basis using offsets measured from a Gaussian fitted water saturation shift referencing (WASSR) spectrum,9 acquired using five Gaussian shaped saturation pulses (same sequence parameters as above except saturation pulse B1 = 0. 5 µT, 25 frequency offsets from -2.0 to 2.0 ppm). Using MATLAB, bulk water, macromolecule, NOE, amide, and amine peaks were fitted to accurately calculate the AACID and NOE effect. In the spinal cord, additional non-saturated scans were interleaved throughout the acquisition and the variation of intensity was used to account for the global effect of respiration.5 The non-saturated scans were spline-interpolated and used to normalize the spectrum. The respiratory cycle was measured with respiratory bellows and used to calculate respiration volume per unit time (RVT). The RVT was scaled by the range of the signal drift of the non-saturated images to regress signal variation due to breathing out of the data.10 One healthy subject was recruited to evaluate CEST effect and the respiratory correction algorithm in the spinal cord.Results
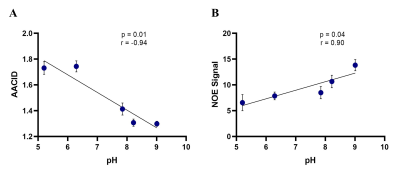
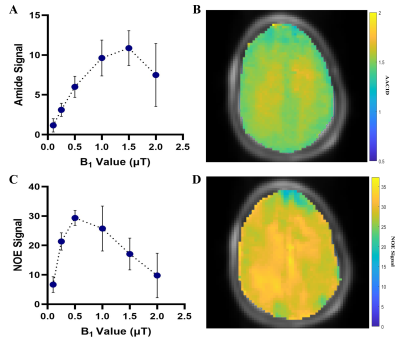
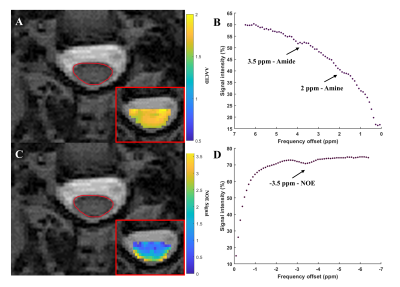
In the egg white phantoms, both the AACID (p = 0.01, r = -0.94) and NOE (p = 0.04, r = 0.90) CEST effects were found to be linearly dependent on pH (Figure 1). Parametric maps of the AACID value and NOE effect in human brain show a homogeneous spatial distribution (Figures 2B and 2D). In the brain, the optimal amide CEST effect was achieved at a B1 value of 1.5 µT and the greatest NOE effect was observed at 0.5 µT (Figure 2). In the spinal cord, the average magnitude of the measured amide CEST effect was 1.34% and the average magnitude of the NOE CEST effect was 2.55% (Figure 3).Discussion
The optimized CEST sequence produced high quality images in the phantom, brain, and spine. The pH dependence of both the AACID value and NOE effects suggested that both could be utilized to investigate pH changes due to hypoxia at this field strength. In the spinal cord, the detected NOE effect was more prominent and could provide an advantage for creation of pH-weighted contrast, also reducing SAR due to the lower B1 amplitude.Conclusion
Although CEST in the spinal cord has been demonstrated previously,10,11 here we examine a prototype CEST sequence at 3T, combined with respiratory correction, to evaluate pH sensitive contrast using both the AACID value and NOE effect. Further optimization in the spinal cord is required to improve the fidelity of the measurements. In the future, the two contrasts will be compared in the spinal cord of healthy subjects to determine the reproducibility of the pH-weighted measurements and in DCM patients to examine the pH heterogeneity at the site of spinal cord compression.Acknowledgements
We thank Scott Charlton and Oksana Opalevych (CFMM, Robarts Research Institute, The University of Western Ontario) for facilitating MRI acquisitions.References
[1] Toledano M, Bartleson JD. Cervical spondylotic myelopathy. Neurol Clin. 2013; 31: 287-305.
[2] Sherry A, Woods M. Chemical exchange saturation transfer contrast agents for magnetic resonance imaging. Annu Rev Biomed Eng. 2008; 10(3/4): 391-411.
[3] van Zijl PCM, Yadav NN. Chemical Exchange Saturation Transfer (CEST): what is in a name and what isn’t? Magn Reson Med. 2011; 65(4): 927-948.
[4] McVicar N, Li AX, Goncalves DF, Bellyou M, Meak SO, Prado MAM, Bartha R. Quantitative tissue pH measurement during cerebral ischemia using amine and amide concentration-independent detection (AACID) with MRI. J Cereb Blood Flow Metab. 2014; 34: 690-698.
[5] Jones CK, Huang A, Xu J, et al. Nuclear Overhauser enhancement (NOE) imaging in the human brain at 7T. NeuroImage. 2013; 77: 114-124.
[6] Zhou J, Payen J, Wilson DA, Traystman RJ, van Zijl PCM. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med. 2003; 9(8): 1085-1090.
[7] Shen Y, Xiao G, Shen Z et al. Imaging of nuclear Overhauser enhancement at 7 and 3 T. NMR Biomed. 2017; 30(9): 1-9.
[8] Paech D, Zaiss M, Meissner JE et al. Nuclear Overhauser Enhancment Mediated Chemical Exchange Saturation Transfer Imaging at 7 Tesla in Glioblastoma Patients. PloS One. 2014; 9(8): 1-7.
[9] Kim M, Gillen J, Landman BA, Zhou J, van Zijl PCM. Water Saturation Shift Referencing (WASSR) for Chemical Exchange Saturation Transfer (CEST) Experiments. Magn Reson Med. 2009; 61: 1441-1450.
[10] By S, Barry RL, Smith AK, et al. Amide Proton Transfer CEST of the Cervical Spinal Cord in Multiple Sclerosis Patients at 3T. Magn Reson Med. 2018; 79: 806-814.
[11] Dula AN, Pawate S, Dethrage LM, Conrad BN, Dewey BE, Barry RL, Smith SA. Chemical exchange saturation transfer of the cervical spinal cord at 7T. NMR Biomed. 2016; 29: 1249-1257.
Figures