2081
Repeatability of B1 Inhomogeneity Correction of Volumetric (3D) Glutamate CEST via High-Permittivity Dielectric Padding at 7T1CAMIPM, Radiology, University of Pennsylvania, Philadelphia, PA, United States, 2Radiology, University of Pennsylvania, Philadelphia, PA, United States, 3Department of Neurology, University of Pennsylvania, Philadelphia, PA, United States, 4Department of Psychiatry, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
Dielectric pads were used to improve the contrast of volumetric (3D) gluCEST via enhanced homogenization of B1 fields. Effective placement of the pads required additional coverage of the face due to their physical size to achieve the saturated B1 strength necessary to recover the gluCEST signal from the anterior portions of the brain. The origin of gluCEST's need for high B1 is discussed, and examples of 3D gluCEST images acquired with and without the use of the dielectric pads are provided and repeatability of the 3D gluCEST in the presence of pad placement was determined.
Introduction
Ultra-high field brain imaging lacks B1 homogeneity due to the shorter RF wavelengths used at higher field strengths in comparison to human head anatomy. Chemical Exchange Saturation Transfer (CEST) methods rely on the B1 field to create endogenous but transient contrast by applying a metabolite-selective saturation pulse. CEST is therefore significantly more vulnerable to B1 inhomogeneity compared to other imaging sequences. Glutamate-weighted CEST (gluCEST) is particularly challenging in this regard because, due to the fast exchange of its proton with surrounding water, it both requires ultra-high field (7T) and high B1 strength (3μT) to generate adequate contrast. In moving from single-slice (2D) imaging to volumetric slabs (3D), the absolute spread of B1 amplitude over the field of view increases and, because one now needs to collect more points in k-space per saturation pulse, the minimum B1 amplitude required to maintain glutamate-derived contrast at an acceptable level increases. In this work, high-permittivity dielectric pads were used to augment the B1 field amplitude during the image acquisition process. While dielectric pads have been adopted in several 7T imaging studies, including NOE and APT CEST measurements1,2,3, it is believed this is the first time that they have been used to improve brain gluCEST imaging at ultra-high field.Theory
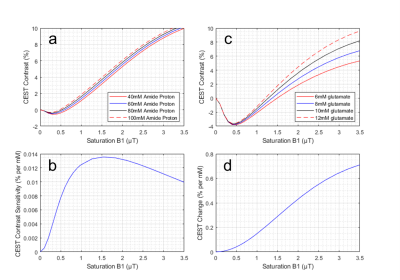
Bloch simulations can allow for an understanding of why sufficient B1 power is necessary for CEST imaging given the proton exchange rate of the metabolite of interest. Figure 1 illustrates that, in contrast to the more widespread technique of APT imaging, accurate gluCEST imaging requires a high and constant B1 power. Figure 1A shows the simulated CEST contrast from saturation at 3.5ppm as a function of B1 strength for different concentrations of amide protons. This illustrates that CEST at 3.5ppm will detect differences in [APT] for a broad range of B1 power, starting at approximately 0.7μT. This sensitivity is quantified in Figure 1B, where the slope of the difference in CEST signal from two concentrations is plotted against the B1 power used. Figure 1C, analogous to Figure 1A, shows CEST contrast at 3.0ppm (gluCEST) and illustrates its ability to detect differences in the concentration of glutamate. In sharp contrast to APT CEST measurements, B1 strengths below 1.5μT are insensitive to glutamate; they almost exclusively reflect differences in water and lipid properties. Figure 1D illustrates that optimal gluCEST contrast can be achieved using the highest possible B1. However, currently, B1 values above 4μT cannot be used in human experiments due to SAR limitations. Dielectric materials with high permittivity values augment existing B1 fields via two main mechanisms. First, the addition of materials to reduce the elliptical shape of tissue volumes can disperse the B1 fields more evenly. Second, displacement currents within the dielectric material create a secondary magnetic field which can add constructively to the primary field from the RF coil4. These materials are typically produced as a powder, with the internal grain size being an important factor in the macroscopic field altering properties. These powders can be mixed into an aqueous solution, as done here, to reduce the T2 value of the solvent and reduce the background MR signal.Methods
All images were acquired on a 7T system (MAGNETOM Terra, Siemens Healthcare, Erlangen, Germany) using a volume transmit 32 channel receive phased-array head coil (Nova Medical, Wilmington, MA, USA). Two subjects were imaged with informed consent under local regulatory supervision. Two dielectric pads were used with a composition of CaTiO3 suspended in D2O (7TNS, Multiwave Imaging, Geneva, Switzerland) and had an estimated permittivity value of 1105. These pads were placed near the temporal lobes on either side of the subject's head, extending forward to partially cover the face (due to physical size). 3D glutamate CEST data was acquired using a modified 3D turbo FLASH sequence, with TR = 3.5ms and TE = 1.79ms, shot TR = 6s. Our 3D slab had a thickness of 24mm (2mm resolution), with an in-plane resolution of 1 x 1mm. Each slice was acquired in three shots (separate saturation/acquisition cycles). GRAPPA was applied with an acceleration factor of 2. Magnetization preparation was achieved using 3μT RMS amplitude, 99ms Hanning shaped pulses with 1ms interpulse delay applied at offset frequencies {±1.8, 2.1, 2.4, 2.7, 3.0, 3.3, 3.6, 3.9, 4.2ppm} relative to water. CEST-weighted images were corrected for the B0 field distribution using WASSR acquisition6 and B1 field maps were generated as in Volz et al7.Results and Conclusion
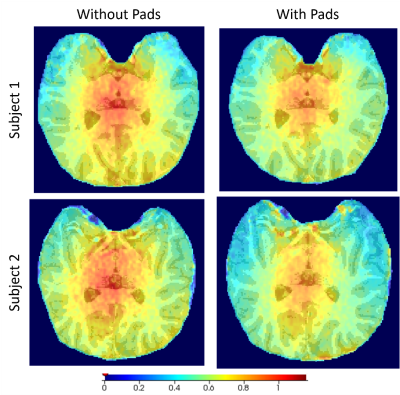
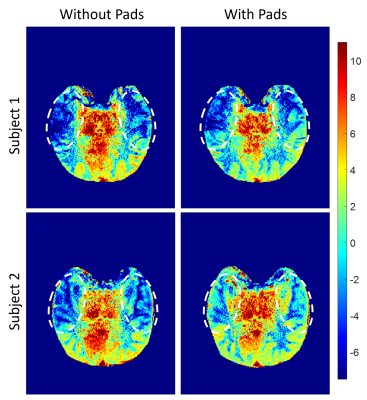
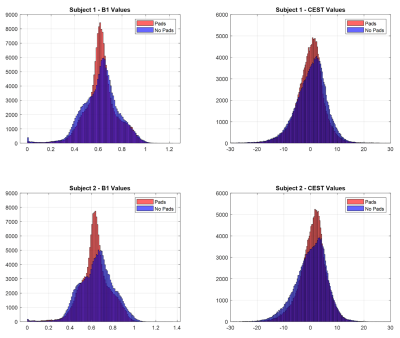
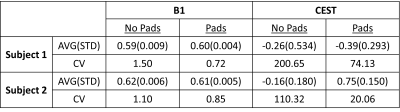
The use of high-permittivity dielectric pads improve the B1 field homogeneity (Figure 2) and enhanced gluCEST contrast (Figure 3) for both subjects when compared to data that did not incorporate pads. Another important aspect of this study was addressing repeatability by showing that improved gluCEST contrast can be reliably achieved between subjects (Figure 4) through careful placement of the pads. Furthermore, Table 1 shows the coefficient of variance (CV) decreasing between pad conditions for both B1 and CEST values, signifying a decrease in variability across each image. No attempt was made to correct for B1 inhomogeneity through post-processing and raw CEST values were corrected only with pads. In the future, integrating B1 post-processing will further improve the data quality.Acknowledgements
Research reported in this work was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under award Number P41EB029460 (RR) and by the National Institute of Aging of the National Institutes of Health under Award Numbers R56AG066656 (DR), R01MH120174 (DR), and R01AG063869 (RR).References
[1] Jones, C. et al. Magn. Reson. Med. 2012: 67(6)
[2] Jones, C. et al. NeuroImage 2013: 77
[3] Heo, H. et al. J. Mag. Res. Im. 2016: 44(1)
[4] Webb, A. Concept Magn. Reson. A. 2011: 38(4)
[5] Teeuwise, W. et al. Magn. Reson. Med. 2012: 67
[6] Kim, M. et al. Magn. Reson. Med. 2009: 61(6)
[7] Volz, S. et al. NeuroImage 2010: 49(4)
Figures