2064
3D MR spirometry sensitivity to gravity lung dependence in healthy volunteers
Nathalie Barrau1, Claire Pellot Barakat1, Zhongzheng He1, Tolga Emre1, Angéline Nemeth1, Anass Afkir1, Wenpei Cai1, Vincent Brulon1, Tanguy Boucneau2, Brice Fernandez2, Florent Besson1, Vincent Lebon1, and Xavier Maître1
1Université Paris-Saclay, CEA, CNRS, Inserm, BioMaps, Orsay, France, 2General Electric Healthcare, Buc, France
1Université Paris-Saclay, CEA, CNRS, Inserm, BioMaps, Orsay, France, 2General Electric Healthcare, Buc, France
Synopsis
Spirometry is a standard exam in pulmonary function testing for assessing the efficiency of global ventilation from flow-volume loops measured at the mouth during forced respiratory cycles. A newly developed technique, 3D MR spirometry, makes use of dynamic magnetic resonance imaging during spontaneous breathing to produce regional characterization of the lung function. The sensitivity of the technique was challenged with respect to gravity by extracting the associated ventilation gradients in prone and supine healthy volunteers.
Introduction
Standard spirometry only provides a global scalar measurement of the pulmonary ventilation. It cannot access the regional function of the organ and is prone to fail for proper diagnostic when the pulmonary alteration is local and blurred in the flow-volume measurement performed at the mouth. Besides, ventilation is essentially a three-dimensional mechanism that can be affected by simply the body position in the Earth gravity field. The above weight promotes more efficient alveolar gas evacuation and increased alveolar density through lung tissue deformation. However, this gravity dependence is usually averaged out over the whole lung1,2.Regional ventilation was recently explored with 3D MR spirometry3. Gravity dependence was used here to challenge the sensitivity of the new technique. It is an established phenomenon, easy to set up, control, and differentiate while the subject is her or his own reference by switching from supine to prone positions. Hence, we carried a preliminary prospective clinical study with 3D MR spirometry onto seven healthy volunteers in supine and prone positions.
Methods
Ten-minute dynamic lung MRI was performed for seven healthy asymptomatic volunteers (3 females, 4 males; 51.5 ± 14.1 y/o; 23.5 ± 3.1 kg/m² of BMI). The volunteers were asked to spontaneously breath while they underwent 4 successive acquisitions: two repeated in supine position and two repeated in prone position. The study was performed at 3T (GE Signa PET/MR scanner) using a UTE sequence combined with an AZTEK trajectory4, and a 30-channel thoracic coil for signal reception. Main parameters were TE = 14 µs, TR = 2ms, flip angle = 3°, bandwidth = 100 kHz, voxel size = 1.5 mm isotropic, matrix = (212×212×142). During the acquisition, the respiratory function was monitored using the outcome of an abdominal belt.A self-navigator was extracted from the center of the acquired k-space to retrospectively rephase the MR data into respiratory phases, resulting into 20 dynamic images of an integrated respiratory cycle over the 10-minute acquisition. The strain tensor was estimated by elastic registration between the images of each temporal phase. Parametric maps characterizing the respiratory function were then derived for each temporal phase: local ventilatory volumes were inferred from the Jacobian of the strain tensor and local flow rates were temporally derived from these local volumes. Ultimately, 3D MR spirometry makes it possible to obtain flow-volume loops in every reconstruction kernel throughout the lung. It yields flow-volume 3D maps and full characterization of the local ventilation.
Analyses were performed into two anterior-posterior identical volumes. The areas of the flow-volume loops of the anterior-posterior regions were computed. Slopes of the mean volume ratios along the gravity direction were computed using a linear fit. These values were compared between acquisitions repeated in the same position and between supine and prone positions. Trends of the gravity dependence and volumes of flow-volume loop area were also compared after rescaling between 0 and 1 to cope for any ventilation variations between scans. Repeatability was evaluated using Bland Altman, and correlation analyses and Intraclass correlation coefficient (ICC). Sensitivity was established upon paired Wilcoxon tests on the supine and prone flow-volume loop area and slope measurements.
Results
The values of the respiratory monitoring vary by 4% in amplitude and 11% in frequency between successive acquisitions with a greater variability in supine position. Volume ratio slopes and flow-volume loop areas are similarly repeatable but flow-volume loop areas are not repeatable in supine. However, rescaled measurements are repeatable (Figure 4).Maximal values of inhaled volume ratios and exhaled flows are found in posterior and anterior regions for supine and prone positions respectively (Figure 1 a,b). The inhomogeneity of the flow-volume maps underlines the gravity dependence of the lung. The dependence is consistently observed on the area of flow-volume loops ($$$p < 10^{-3}$$$) (Figure 1 c and Figure 4). The anterior-posterior slope also differs ($$$p < 10^{-3}$$$) (Figure 4). Finally, rescaled parameters show strong statistical difference between dependent and nondependent regions (Figure 4).
Discussion
Three-dimensional MR spirometry is moderately repeatable before parameters are rescaled possibly because monitored respiratory breathing conditions and patterns evolve from one acquisition to another. Simultaneous standard spirometry measurements could account for physiological variations in spontaneous breathing and a more elaborated approach than rescaling, implemented here, could be used to produce quantitative repeatable measurements. The ventilation gradient is stronger in supine than in prone essentially because the mediastinum applies a greater force on the parenchyma5,6. This gradient is reversed in the most dependent regions at the periphery of the lung where alveoli are expected to remain deflated7.Conclusion
Three-dimensional MR spirometry is fairly repeatable when the measurement outcomes are properly rescaled. The technique is sensitive to gravity effects either in supine or prone positions whereas the subject is spontaneously breathing. Ventilation is less governed by gravity and it more homogeneously distributed in the lung in prone position.Acknowledgements
Peder Larson for UTE sequence. The PET/MR platform is affiliated to the France Life Imaging network (grant ANR-11-INBS-0006).References
1. Bryan, A. C., Milic-Emili, J., & Pengelly, D. (1966). Effect of gravity on the distribution of pulmonary ventilation. Journal of Applied Physiology, 21(3), 778-784.Bryan Emili2. Hopkins, S. R., Henderson, A. C., Levin, D. L., Yamada, K., Arai, T., Buxton, R. B., & Prisk, G. K. (2007). Vertical gradients in regional lung density and perfusion in the supine human lung: the Slinky effect. Journal of applied physiology, 103(1), 240-248.
3. Boucneau, T., Fernandez, B., Larson, P., Darrasse, L., & Maître, X. (2020). 3D magnetic resonance spirometry. Scientific Reports, 10(1), 1-12.
4. Boucneau, T., Fernandez, B., Besson, F. L., Menini, A., Wiesinger, F., Durand, E., ... & Maître, X. (2021). AZTEK: Adaptive zero TE k‐space trajectories. Magnetic Resonance in Medicine, 85(2), 926-935.
5. Henderson, A. C., Sá, R. C., Theilmann, R. J., Buxton, R. B., Prisk, G. K., & Hopkins, S. R. (2013). The gravitational distribution of ventilation-perfusion ratio is more uniform in prone than supine posture in the normal human lung. Journal of applied physiology, 115(3), 313-324.
6. Klimeš, F., Voskrebenzev, A., Gutberlet, M., Kern, A., Behrendt, L., Kaireit, T. F., ... & Vogel‐Claussen, J. (2019). Free‐breathing quantification of regional ventilation derived by phase‐resolved functional lung (PREFUL) MRI. NMR in Biomedicine, 32(6), e4088.
7. Tobin, A., & Kelly, W. (1999). Prone ventilation—it's time. Anaesthesia and intensive care, 27(2), 194-201.
Figures
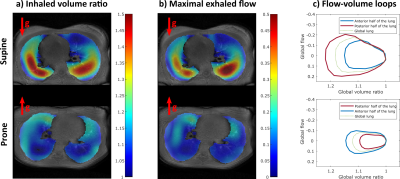
3D spirometry in one healthy volunteer over an integrated respiratory cycle: a) Map of inhaled volume ratio in a central axial slice b) Map of maximal exhaled flow during an integrated respiratory cycle in a central axial slice c) Flow-volume loops averaged over the anterior and posterior halves of the lung and over the overall lung.
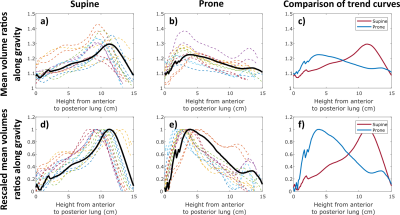
Averaged inhaled volume ratios along the gravity axis for supine and prone positions before (a to c) and after rescaling (d to f). For plots a) b) d) and e), the colored curves represent the results obtained for each volunteer and the black curves an inter-individual average.
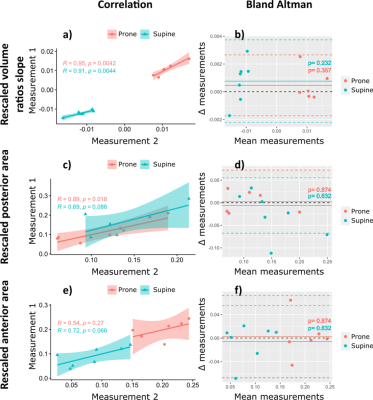
Correlation
(a, c and e) and Bland Altman (a, d and f) analysis evaluating consistency and
agreement of two successive measurements in prone and supine positions
Statistics
table for gravity lung dependence and measurement repeatability
DOI: https://doi.org/10.58530/2022/2064