2062
Choreography Controlled (ChoCo) by synchronized video for motion artifact reproducibility and dataset generation
Oscar Albert Dabrowski1, Sébastien Courvoisier2, Jean-Luc Falcone3, Antoine Klauser2, Julien Songeon3, Michel Kocher2, Bastien Chopard3, and Francois Lazeyras2
1Computer science, University of Geneva, Geneva, Switzerland, 2CIBM, Geneva, Switzerland, 3University of Geneva, Geneva, Switzerland
1Computer science, University of Geneva, Geneva, Switzerland, 2CIBM, Geneva, Switzerland, 3University of Geneva, Geneva, Switzerland
Synopsis
In the age of artificial intelligence, there is a need for simple and inexpensive frameworks aimed at performing standardized reproducible motion-controlled experiments allowing the creation of datasets of motion corrupted in vivo MRI scans. Focused on human brain imaging, we propose ChoCo: a choreography controlled protocol for reproducible head motion and an ad-hoc hardware device for synchronization between an MRI scanner and a movie displaying motion to be performed. A proof of concept demonstrated that 6 participants were able to reproduce head choreographies accurately. The resulting motion corrupted brain images show qualitatively similar artifacts, confirming consistent motion reproduction among subjects.
Introduction
We propose a protocol and a simple device allowing choreography control by video (ChoCo) for head motion data acquisition in a standardized and reproducible way. In the context of understanding the complex problem of the effects of motion in MRI1, ChoCo would provide means to collect corrupted labelled MRI datasets. The control of the head choreographies is done by a simple setup: a moving target shown to the subject on a screen combined with customized plastic glasses with a pinhole are constraining the tracking2 process by the subject’s head. External synchronization between the stimuli and standard MRI sequences is done by an ad hoc device.Such a system without known competitors in the literature is compatible with any pulse sequence and clinical MRI scanner, provided that a video stimuli system is available.
Methods
System setup: head choreographies were controlled by a movie displayed on a screen at the end of the MRI bore. A moving dot shows to the subjects the motions to be performed. Each participant was wearing customized plastic glasses with a pinhole to allow dot tracking by the head and avoid eye-tracking motions. The process is analogous to following a moving target with binoculars (Fig. 1). For a proof of concept, two rotational motion types were chosen: abrupt (i.e., short back and forth motion) and ‘’motion and hold’’ (i.e., pause of a few seconds before going back to initial position). Both types of motion were performed in two directions: namely right/left (R/L) and up/down (U/D), which correspond to yaw and pitch rotation directions, respectively (considering the convention illustrated in Fig. 1). Precise timing and synchronization between the pulse sequence and the choreography stimuli was obtained either with the TR output signal of the EPI pulse sequences (fMRI type) or with an external device (Arduino, with optical output) using the external trigger option available on the MRI scanner (see Fig. 2 for setup overview). Six volunteers performed the head choreographies on a 3T MRI scanner (Prisma fit, Siemens).Technical parameters: multi-volume (>600) multislice (36 slices/volume) EPI SMS pulse sequences (TE = 30 ms, TR = 600 ms, slice acceleration factor = 4) with 3x3x3.3 mm voxels were used for motion estimation. Classical 2D Spin-Echo (TE = 12 ms and TR = 750 ms) and 3D T1 MP-RAGE (TE = 2.36 ms and TR = 1930 ms) were performed to propose samples of motion artifacts for commonly used clinical pulse sequences. Spin-Echo and MP-RAGE sequences were externally triggered with an acquisition window of 800 and 1950 ms, respectively. Voxel dimensions of 0.9x0.9x4.0 mm and 0.9 mm isotropic were used for SE and MP-RAGE, respectively.
Data postprocessing: EPI volumes were realigned using the SPM software3 to estimate rigid-body motion parameters. Average subject angle error with respect to target was measured as well as subject stability (baseline standard deviation).
Results
Subjects are able to reproduce head motion choreographies accurately (Fig. 3) even if subjects may deviate from the baseline (0° angle). The effective angles were computed with respect to the local baseline. The training sessions (5 repetitions of two different motion types) also show that a learning process takes place, usually after about 2 repetitions. Indeed, the angle amplitudes get closer to targets even before the end of the sessions. Moreover, a sliding window standard deviation computed over each subject’s baseline confirms that subjects are able to maintain the latter reasonably stable (with again a slight decrease of the standard deviation near the end of the training session).Spin-Echo and MP-RAGE acquisitions show that the motion artifacts reproduced across subjects are qualitatively similar, which argues strongly in favour of accurate motion reproduction among all subjects after the training phase. In Fig. 5, a Spin-Echo k-space magnitude (averaged over 27 slices, for the same subject), reconstructed with the ESPIRiT algorithm4, exhibits dark bands5 at phase columns corresponding to motion onset.
Discussion
Accurate motion reproduction demonstrates the usefulness of our approach, which is simple, universal and can be modified to include other types of motion. Results pertaining to training data encourages the use of this preliminary session before performing the actual representative scan. Given the results we obtained, we are confident that with a reasonable training session (less than 6 minutes), following the ChoCo protocol, subjects will be able to reproduce the head choreographies accurately. The qualitative similarity of motion artifacts in Spin-Echo and T1 3D MP-RAGE, for all subjects, was expected given correct head choreography reproduction and correct timing was further confirmed by visual inspection of classical Spin-Echo k-space magnitude (Fig. 5) with respect to projected movie.Conclusion
Our work proposes a simple setup and protocol to perform standardized motion-controlled experiments allowing researchers in the MRI community to study the effects of well controlled motion on any MRI pulse sequence without modification or complex external setup.Acknowledgements
No acknowledgement found.References
- Maclaren, J., Armstrong, B.S., Barrows, R.T., Danishad, K.A., Ernst, T., Foster, C.L., Gumus, K., Herbst, M., Kadashevich, I.Y., Kusik, T.P. and Li, Q., 2012. Measurement and correction of microscopic head motion during magnetic resonance imaging of the brain. PloS one, 7(11), p.e48088.
- Maclaren, J., Herbst, M., Speck, O. and Zaitsev, M., 2013. Prospective motion correction in brain imaging: a review. Magnetic resonance in medicine, 69(3), pp.621-636.
- Penny, W.D., Friston, K.J., Ashburner, J.T., Kiebel, S.J. and Nichols, T.E. eds., 2011. Statistical parametric mapping: the analysis of functional brain images. Elsevier.
- Uecker, M., Lai, P., Murphy, M.J., Virtue, P., Elad, M., Pauly, J.M., Vasanawala, S.S. and Lustig, M., 2014. ESPIRiT—an eigenvalue approach to autocalibrating parallel MRI: where SENSE meets GRAPPA. Magnetic resonance in medicine, 71(3), pp.990-1001.
- Wood, M.L., Shivji, M.J. and Stanchev, P.L., 1995. Planar‐motion correction with use of k‐space data acquired in Fourier MR imaging. Journal of Magnetic Resonance Imaging, 5(1), pp.57-64.
Figures
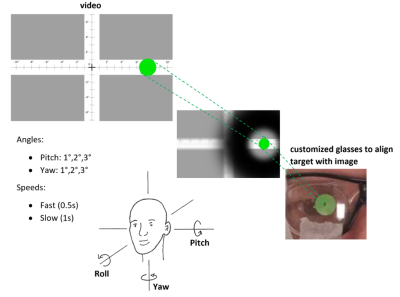
Figure 1 Experimental setup showing
customized plastic glasses with a pinhole to avoid eye-tracking by restricting
subject’s field of view. The volunteer must keep the green dot in the center of
the pinhole at all times.
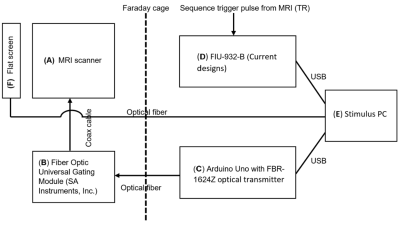
Figure 2 Diagram showing the hardware
setup and the interconnection of the different components.
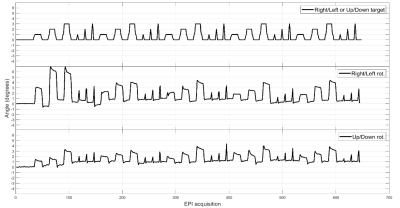
Figure 3 Typical head motion
choreography results obtained for subject#2 after EPI realignment
(w.r.t. first volume) with SPM. The first row shows the target and the two
other rows below show the effective results obtained for this subject.
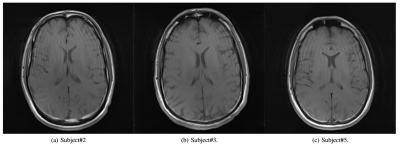
Figure 4 Representative results of
motion artifacts obtained for 3 different subjects (Spin-Echo acquisition,
axial slices). Ringing-like artifacts can be observed at the same locations for
each subject with a qualitatively similar spacing (spatial frequency).
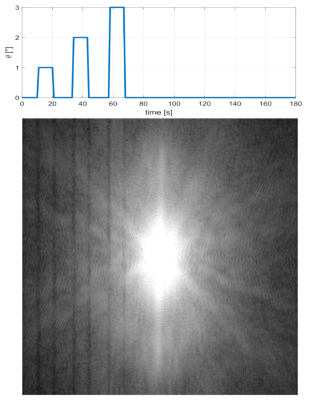
Figure 5 Mean k-space magnitude
obtained for 27 slices of a Spin-Echo acquisition for one subject. Vertical
dark lines are visible at positions corresponding to motion blocks. The graph
above shows how motion occurrences closely match k-space corruption lines (with
respect to motion timing from head choreography movie).
DOI: https://doi.org/10.58530/2022/2062