2059
Prospective Motion Correction for a Full Neuroimaging Protocol in Elderly Subjects at 7T1Stanford University, Stanford, CA, United States, 2GE Healthcare, Stanford, CA, United States, 3Weill Cornell Medicine, New York City, NY, United States, 4Hobbitview Inc., San Jose, CA, United States
Synopsis
Correction of head motion during MRI scanning is critical to achieving high-resolution images to study and quantify small regions such as the hippocampus in Alzheimer’s disease. Our optical prospective motion correction prototype system has previously demonstrated improved image quality in volunteers at 7T, but clinical translatability has been lacking. In this work, we implemented an integrated power supply and applied prospective correction to a broad set of sequences, including mGRE and FSE, for a clinically relevant research protocol with emphasis on hippocampal iron imaging. Metrics achievable using our protocol include high-resolution cortical QSM, hippocampal subfield QSM and volume.
Introduction
While high-field MRI can generate higher resolution images than conventional field strengths, such high-resolution imaging requires longer scan times, and the process is highly susceptible to corruption from motion artifacts1-3. However, this obstacle must be overcome because small brain regions such as the hippocampus area are of high interest in Alzheimer’s disease and other neurologic disorders. We have published a proof-of-concept paper on our optical prospective motion correction system4-6. This work had been performed on a subset of representative sequences. We had also leveraged an MR-safe external battery source to power the camera which had finite life and set a limit on maximum scan time. Here, we build on our previous work to: a) develop an integrated power source, b) extend motion correction to other sequences, including high-resolution mGRE/QSM, FSE using parallel acceleration, and c) demonstrate use of this clinically relevant research protocol on control subjects.Methods
The prospective tracking system consisted of an MR‐compatible camera, a marker, and a tracking computer, designed and built to be mounted between the Tx and Rx coils of a Nova Medical 2 Tx/32 Rx head coil on a 7T GE MR950 scanner. This set-up enables direct line-of-sight to an MR-safe, checkerboard-marker placed with adhesive on the subject’s forehead to record subject head motion during scanning.We powered the camera using a cable integrated into the scanner bed. We utilized the spare 15V pins on the 7T GE MR950 scanner and attached a coaxial cable connector to extend this DC power from the RF connector plane to the camera. Cable traps were placed every 30cm along this twisted-pair extension cable, each consisting of a pair of L-151 inductors with 300MHz self-resonance frequency, and a series-connected 7th-order Butterworth low-pass filter, were added to suppress high frequency interference.
Expanding our range of motion correction-enabled sequences and building a clinically applicable research protocol for high-resolution brain imaging, we scanned a healthy, compliant 67-year-old and 48-year-old male subjects with IRB approval while they were instructed to hold as still as possible with the following protocol. For each sequence, on another set of volunteers, we validated by testing with/without motion and with/without correction that correction always improved image quality.
- 2D gradient echo (2D-Fast) (0.1758x0.1758x1.0mm resolution, TR=300ms, TE=20ms, FA=35°, 6 slices spaced at 6mm) 4-5 repetitions.
- T2-weighted fast spin echo (FSE) (512x512x38, 0.33x0.33x1.5mm, TR=9375ms, TE=50.88ms, cardiac gating on, FC slice[MZ1] , ARC=2, 8 echoes) for hippocampal subfield segmentation. We utilized ARC to accelerate while ensuring the coil sensitivity profile would compensate for head motion as well.
- T1-weighted BRAVO (256x256x124, 0.94x0.94x1.0mm, TR=9.2ms, TE=3.116ms, FA=10°)
- Iron-sensitive QSM sequences: one to generate whole brain coverage with isotropic voxels (0.86mm isotropic, TR=29.40ms, TE=1.95-15.5ms, 8 echoes, ARC=2x2), and one coronal obliqued to achieve super high-resolution in-plane coverage of the hippocampus (512x512x58, 0.43x0.43x1mm, TR=34.10ms, TE=3.67-18.5ms, 5 echoes, ARC=2x2).
Results
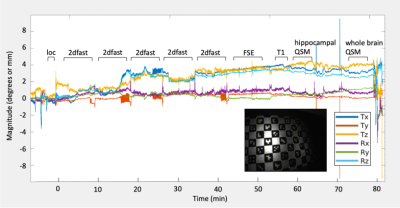
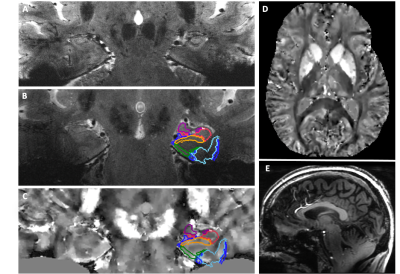
Images acquired were of high quality and lacked motion artifacts that would otherwise be seen in non-motion corrected sequences. Fig. 1 shows continuous motion plots throughout the entire exam, illustrating that although the subject was instructed to remain still, there was still over 4° and 4mm of head movement throughout the 1-hour exam. Fig. 2A shows the resulting high-resolution 2D-Fast image, depicting detailed hippocampal microanatomy and iron sensitivity. The high-resolution FSE and BRAVO sequences enable an automated hippocampal subfield segmentation based on a 7T brain atlas (Fig. 2B,E). High-resolution hippocampal and whole brain QSM images are shown in Fig. 2C-D, with subfield segmentations overlaid, permitting subfield susceptibility quantification. Subfield QSM quantitation from the 67-year-old subject is within the range previously reported9 (Table 1).Discussion/Conclusion
Correction of involuntary motion during a multi-sequence brain exam was successfully demonstrated on healthy human subjects, producing high-quality anatomic imaging as well as quantitative maps including R2* and QSM images at 7T. The corrected acquisitions were consistent with what we have seen in our individually validated images, now shown throughout an entire corrected exam.Our motion-correction system demonstrates the potential to overcome motion artifact throughout an entire brain exam (>1hr) at 7T, one of the biggest challenges precluding the utilization of ultra-high-field MR for high-resolution and quantitative brain imaging. This system allows for ultra-high-resolution anatomic imaging and QSM and R2* mapping, which may facilitate discovery of novel disease-related image-based biomarkers in the brain. Motion artifact was present even in healthy, compliant subjects, demonstrating the susceptibility of these sequences to motion and underscoring the importance of this approach. We hope to utilize this motion correction system and quantitative brain examination across a range of ages and disease conditions in future work.
Acknowledgements
The authors would like to acknowledge research support by GE Healthcare, NSF GRFP DGE – 1656518, and NIH 1R01AG061120-01.References
1. Mattern H, Sciarra A, Lüsebrink F, Acosta‐Cabronero J, Speck O. Prospective motion correction improves high‐resolution quantitative susceptibility mapping at 7T. Magnetic Resonance in Medicine. 2018.
2. Stucht D, Danishad KA, Schulze P, Godenschweger F, Zaitsev M, Speck O. Highest Resolution In Vivo Human Brain MRI Using Prospective Motion Correction. PLoS One. 2015.
3. Mattern H, Sciarra A, Godenschweger F, Stucht D, Lüsebrink F, Rose G, Speck O. Prospective motion correction enables highest resolution time‐of‐flight angiography at 7T. Magnetic Resonance in Medicine. 2018.
4. DiGiacomo P, Tong E, Maclaren J, Aksoy M, Bammer R, Rutt B, Zeineh M. A novel, coil-integrated camera for prospective optical motion correction of brain imaging at 7T. International Society of Magnetic Resonance in Medicine. 2019, Montreal, Canada.
5. DiGiacomo P, Maclaren J, Aksoy M, Tong E, Carlson M, Lanzman B, Hashmi S, Watkins R, Rosenberg J, Burns B, Skloss TW. A within‐coil optical prospective motion‐correction system for brain imaging at 7T. Magnetic Resonance in Medicine. 2020.
6. DiGiacomo P, Carlson M, Maclaren J, Aksoy M, Wang Y, Spincemaille P, Burns B, Bammer R, Rutt B, Zeineh M. Optical prospective motion correction enables improved quantitative susceptibility mapping (QSM) of the brain at 7T. International Society of Magnetic Resonance in Medicine. 2020, virtual.
7. Liu J, Liu T, de Rochefort L, Ledoux J, Khalidov I, Chen W, Tsiouris AJ, Wisnieff C, Spincemaille P, Prince MR, Wang. Morphology enabled dipole inversion for quantitative susceptibility mapping using structural consistency between the magnitude image and the susceptibility map. Neuroimage. 2012.
8. Liu Z, Kee Y, Zhou D, Wang Y, Spincemaille P. Preconditioned total field inversion (TFI) method for quantitative susceptibility mapping. Magnetic Resonance in Medicine. 2017.
9. Goubran M, Rudko DA, Santyr B, Gati J, Szekeres T, Peters TM, Khan AR. In vivo normative atlas of the hippocampal subfields using multi‐echo susceptibility imaging at 7 Tesla. Human brain mapping. 2014 Aug;35(8):3588-601.
Figures