2057
Magnetic Resonance Thermometry Motion Compensation during Focused Ultrasound Controlled Hyperthermia in a Small Animal Model1Biomedical Engineering, University of Toronto, Toronto, ON, Canada, 2Neuroscience and Mental Health, The Hospital for Sick Children, Toronto, ON, Canada, 3Institute of Medical Sciences, University of Toronto, Toronto, ON, Canada, 4Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, ON, Canada, 5Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada, 6Radiation Physics, University Health Network, Toronto, ON, Canada
Synopsis
Magnetic resonance guided high intensity focused ultrasound (MRgHIFU) has gained interest over the past decade due to its ability to administer controlled hyperthermia for localized drug release. One of the main challenges is that MR thermometry is highly susceptible to motion artifacts. A hybrid principal component analysis and projection onto dipole fields motion artifact removal method was applied in real-time during controlled hyperthermia in a murine model using a small-animal MRgHIFU system (Bruker 7T MRI and IGT HIFU). For a target temperature of 40.5°C to be maintained, a significant increase in ultrasound power was required when tissue motion was observed.
Introduction
High intensity focused ultrasound (HIFU) has made significant advances as a cancer therapy, both as a thermal ablation therapy and for drug delivery applications1. Localized mild-hyperthermia of temperatures from 40-42°C can cause targeted drug release from thermosensitive liposomes2. The narrow temperature range is due to the specific formulation of thermosensitive liposomes required for drug release and that temperatures above 43°C can reduce perfusion which impedes drug uptake3. Accurate temperature measurements are crucial as drug release efficacy decreases substantially if the target temperature is not achieved. Efficacy drops from 80% at 40°C, to 60% at 39°C and further down to 25% at 38°C4.Image guidance and monitoring of HIFU using magnetic resonance imaging (MRgHIFU) allows for real-time, high resolution thermal maps, which provide a method to control thermal delivery and evaluate therapy accuracy at the treatment point1. Our custom hyperthermia software, Proteus, controls tumor temperature to ±1°C based off real-time thermometry using a proportional integral derivative (PID) controller5. One of the main challenges with magnetic resonance (MR) thermometry is that it is highly susceptible to motion artifacts which confounds temperature measurements and can skew temperature readings6. This often leads to overestimation of thermal signals as current thermometry algorithms have no way of differentiating temperature from motion, resulting in both being displayed as thermal signal. If motion does occur, Proteus will interpret the artifacts as heating and lower sonication power levels accordingly, which may result in underheating the tissue. Real-time motion compensation algorithms can reduce temperature uncertainty during treatment by adjusting for motion as it occurs, allowing Proteus to deliver more accurate levels of sonication. The motion artifact removal algorithm employed in this work is a hybrid method of principal component analysis and projection onto dipole fields (PCA-PDF)7. Where the PCA and PDF components of the algorithm compensates for periodic and sporadic motion, respectively7. The objective of this work is to demonstrate the ability of a real-time PCA-PDF motion compensation algorithm to negate motion artifacts from MR thermometry obtained from hyperthermia treatments in a murine model.
Methods
Healthy immunocompetent mice (n=6) received 5 minutes of controlled hyperthermia in the hindlimb without PCA-PDF, followed by a 5-minute cooling period before a sequential 5-minute controlled hyperthermia with real-time PCA-PDF. An atlas of 20 images were acquired during pre-heating for the PCA-PDF algorithm to reference. The hyperthermia was administered using a dedicated small-animal MRgHIFU system consisting of a 7T Bruker MRI (70/30 BioSpec, Bruker, Ettlingen, Germany) and Image Guided Therapy (IGT) HIFU device (LabFUS, Image Guided Therapy, Pessac, France). Our software for MRgHIFU procedures (Proteus) monitored the temperature in a region of interest (ROI) set in the hindlimb and adjusted sonication levels to maintain an average ROI temperature of 40.5°C over the treatment. A drift tube marked with a second ROI in Proteus measured any MR bore temperature shift over time. Rectal and esophageal probes were used to monitor core body temperatures.Retrospective PCA-PDF analysis was completed on the hyperthermia sessions without motion compensation. The sonication power over the treatment was analyzed by calculating the spatial average temporal average intensity (ISATA). A paired t-test was performed for both the average temperatures and ISATA values between the controlled hyperthermia with and without the PCA-PDF algorithm, where a p-value < 0.05 is deemed statistically significant.
Results
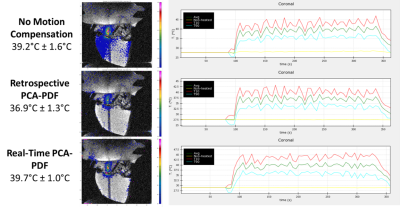
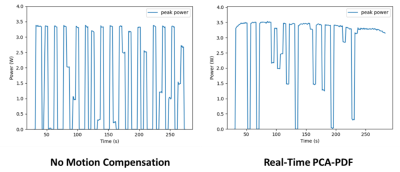
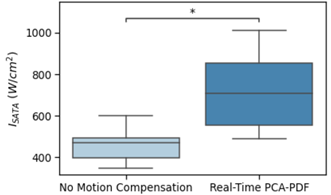
There was a statistical difference between the average ISATA values for the controlled hyperthermia administered on mice with no motion compensation and real-time PCA-PDF, at 424W/cm2 and 683W/cm2, respectively.Even with the significant difference in sonication power, hyperthermia sessions both with and without the real-time PCA-PDF were able to maintain a steady target temperature. Over the 5-minute hyperthermia treatment without motion compensation, the average temperature was 39.6°C ± 1.3°C for the mice. Retrospective PCA-PDF analysis was completed on these hyperthermia treatments with no motion compensation, where the average temperature decreased significantly after motion artifact removal to 37.4°C ± 1.1°C. The hyperthermia treatments with the real-time PCA-PDF motion compensation algorithm had an average temperature of 39.6°C ± 1.0°C.
Discussion
There is a significant increase in ISATA when the PCA-PDF algorithm is applied in real-time in comparison to when no motion compensation is used. This along with the retrospective analysis, suggests that without the PCA-PDF algorithm, temperatures are overestimated due to motion, resulting in reduced sonication power and underheating the target tissue. It is imperative to sufficiently heat the tissue, as temperatures even 1°C below the target temperature can decrease drug release by 20%.The execution of the real-time PCA-PDF algorithm had no effect on the performance of the PID controller capabilities to maintain a target temperature to +/- 1°C. The main difference between the controlled hyperthermia with and without the PCA-PDF motion compensation algorithm is the sonication power required to maintain the target temperature of 40.5°C.
Conclusion
In conclusion, ideal hyperthermic temperatures can be maintained with more confidence that motion isn’t overestimating the temperature when using the PCA-PDF algorithm in real-time. By removing any motion artifacts present, the PID controller required significantly higher levels of sonication power to reach the same target temperature in the hindlimb of the mice. Future work includes investigating whether implementing the real-time PCA-PDF algorithm leads to increased drug release in vivo.Acknowledgements
This work was supported by the Natural Sciences and Engineering Research Council of Canada and a C17 Children's Cancer & Blood Disorders Research Grant.References
1. Tempany CMC, McDannold NJ, Hynynen K, Jolesz FA. Focused ultrasound surgery in oncology: Overview and principles. Radiology. 2011;259(1):39-56. doi:10.1148/radiol.11100155
2. Yarmolenko PS, Zhao Y, Landon C, et al. Comparative effects of thermosensitive doxorubicin-containing liposomes and hyperthermia in human and murine tumours. Int J Hyperth. 2010;26(5):485-498. doi:10.3109/02656731003789284
3. Rossmanna C, Haemmerich D. Review of temperature dependence of thermal properties, dielectric properties, and perfusion of biological tissues at hyperthermic and ablation temperatures. Crit Rev Biomed Eng. 2014;42(6):467-492. doi:10.1615/CritRevBiomedEng.2015012486
4. Dunne M, Epp-Ducharme B, Sofias AM, Regenold M, Dubins DN, Allen C. Heat-activated drug delivery increases tumor accumulation of synergistic chemotherapies. J Control Release. 2019;308(June):197-208. doi:10.1016/j.jconrel.2019.06.012
5. Zaporzan B, Waspe AC, Looi T, Mougenot C, Partanen A, Pichardo S. MatMRI and MatHIFU: Software toolboxes for real-time monitoring and control of MR-guided HIFU. J Ther Ultrasound. 2013;1(1). doi:10.1186/2050-5736-1-7
6. Rieke V, Pauly KB. MR thermometry. J Magn Reson Imaging. 2008;27(2):376-390. doi:10.1002/jmri.21265
7. Tan J, Mougenot C, Pichardo S, Drake JM, Waspe AC. Motion compensation using principal component analysis and projection onto dipole fields for abdominal magnetic resonance thermometry. Magn Reson Med. 2019;81(1):195-207. doi:10.1002/mrm.27368
Figures