2050
Precuneus dysconnectivity with heart failure
Karsten Mueller1, Harald E Möller1, Birol Taskin1, Friederike Thiel1,2,3, Frank Beutner3,4, Andrej Teren3,4, Arno Arno Villringer1,2,3, and Matthias L Schroeter1,2,3
1Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 2Clinic for Cognitive Neurology, University Hospital Leipzig, Leipzig, Germany, 3Leipzig Research Center for Civilization Diseases, Leipzig, Germany, 4Leipzig Heart Center, Leipzig, Germany
1Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 2Clinic for Cognitive Neurology, University Hospital Leipzig, Leipzig, Germany, 3Leipzig Research Center for Civilization Diseases, Leipzig, Germany, 4Leipzig Heart Center, Leipzig, Germany
Synopsis
Our investigations identified the precuneus as the brain region showing a major involvement in connectivity decline in patients with heart failure. Subsequent seed-based correlation analysis showed decreased putamen connectivity related to decline in cognitive performance. In line with these findings and with current Alzheimer’s Disease models showing precuneus connectivity decline as a key feature of brain degeneration, there might be common underlying mechanisms leading to brain connectivity decrease in heart failure and dementia. This hypothesis would be also supported by specific network centrality alterations between precuneus and widely distributed cortical regions particularly in patients showing reduced cognitive performance.
Introduction
Apart from the heart, the brain is most sensitive to acute or subacute distortions of its supply with blood1,2. Recent works describe structural brain damage with heart failure1,3, however, not much is known about alterations of brain function and connectivity. Current literature4 describes cognitive deficits related to heart failure, but a potential link to decline in cognitive and brain function is still missing. Therefore, the aim of the current study was to detect functional correlates of heart failure in terms of alterations in functional brain connectivity. In particular, we were interested in potential connectivity decline related to reduced cognitive performance.Methods
A group of 80 patients (22 females; age: 54.9±5.3 years; mean±SD) was included in the study. Participants were grouped into patients with coronary artery disease (CAD; N=58; 14 females, age: 54.6±5.4 years) and subjects diagnosed with no abnormality (NoAb; N=22; 8 females; age: 55.7±5.0 years). Patients with CAD were further grouped into patients with and without heart failure (CAD+ and CAD-, respectively) according to guidelines of the European Society of Cardiology.Upon hospitalization, initial clinical parameters were obtained including cardiac ejection fraction (EF) and serum concentrations of N-terminal fragment of brain natriuretic peptide (NT-proBNP). After 3.5±1.3 years, EF and NT-proBNP were again obtained in a follow-up session that also included cognitive testing and MRI data acquisition (3T MAGNETOM Verio, Siemens Healthineers, Erlangen, Germany). Functional MRI was performed in “resting state” using gradient-echo EPI (TR=2000ms; TE=30ms; FA=90°; bandwidth 1953 Hz/pixel; acquisition matrix 64×64 pixels; 30 axial slices; slice thickness 4mm; 0.8mm gap; nominal image resolution 3×3×4.8mm3; 300 repetitions; total acquisition time 10min).
Brain network centrality was computed within the CONN toolbox5 using global correlation (GCOR). The following three GCOR-group comparisons were performed using two-sample t-tests including age, sex, and BMI as additional nuisance regressors: “CAD+ vs CAD-”, “CAD+ vs NoAb”, and “CAD+ vs ALL-”, where ALL- is the group of all patients showing no heart failure (CAD- and NoAb). In addition, correlation analyses were performed across all participants to investigate a potential relationship between GCOR and EF, and between GCOR and NT-proBNP, using both the initial and the follow-up measurements.
For all participants, seed-based correlation maps were generated using the precuneus as seed-region. Group analysis was performed using a flexible factorial model with factors heart failure and cognitive performance. Statistical analysis was aimed at a potential interaction between both factors including subsequent post-hoc tests. All statistical parametric maps were generated with P<0.001, and significant clusters were obtained with P<0.05 using FWE correction at cluster-level.
Results
With all three group comparisons, a significant GCOR decrease was found in the CAD+ group specifically in the precuneus (Fig. 1). The inverse comparisons did not show any GCOR increase in CAD+.A significant positive correlation was found between GCOR and the initial EF-value in the precuneus (Figs. 2A,B), however, no significant positive correlation was found between GCOR and the follow-up EF-measurement after FWE-correction (Fig. 2C). Using both the initial and the follow-up measurement of NT-proBNP, a significant negative correlation between GCOR and NT-proBNP was obtained in the precuneus (Fig. 3).
Seed-based connectivity analysis showed a significant interaction between heart failure and cognitive performance (Fig. 4C) related to a significant decrease of precuneus connectivity with reduced cognitive performance in heart failure (Fig. 4A).
Discussion
In line with recent work6, our investigations identified the precuneus as the brain region showing a major involvement in connectivity decline in patients with heart failure. In addition, this centrality decrease was found to be associated with heart failure-related biomarkers, such as EF and NT-proBNP. Seed-based correlation analysis showed decreased putamen connectivity related to decline in cognitive performance.Considering potential neuropathophysiological mechanisms leading to decline in brain connectivity with heart failure, our findings should be seen in the context of the heart’s pump efficiency and changes in the vascular system, leading to alterations in cerebral blood flow (CBF). Current findings support the hypothesis that cardiac dysfunction impacts CBF that would be related to cognitive function4. An underlying mechanism might be due to chronic hypoperfusion in major brain regions leading to injury7. Interestingly, reduction in regional CBF was detected specifically in the precuneus in heart failure8 and patients with Alzheimer’s disease (AD)9. Cerebral hypoperfusion (and hypometabolism) is a well-known feature in AD with the precuneus as the first affected region10,11 that is also supported by a meta-analysis in AD prediction12. Our heart failure-related findings are located in exactly the same precuneus region that lends support to an interpretation of our findings also in the context of AD.
Conclusion
In line with current AD models showing precuneus connectivity decline as a key feature of brain degeneration, there might be common underlying mechanisms leading to brain connectivity decrease in heart failure and AD. This hypothesis would be also supported by specific network centrality alterations between precuneus and widely distributed cortical regions particularly in patients showing reduced cognitive performance.Acknowledgements
Supported by LIFE – Leipzig Research Center for Civilization Diseases, University Leipzig. LIFE is funded by means of the European Union, by the European Regional Development Fund (ERDF) and by means of the Free State of Saxony within the framework of the excellence initiative. AV and MLS have been supported by the German Research Foundation (DFG; CRC 1052 to AV, and SCHR 774/5-1 to MLS).References
- Mueller K, Thiel F, Beutner F, et al. Brain damage with heart failure: Cardiac biomarker alterations and gray matter decline. Circ Res. 2020; 126: 750-764.
- Horstmann A, Frisch S, Jentzsch RT, et al. Resuscitating the heart but losing the brain: Brain atrophy in the aftermath of cardiac arrest. Neurology. 2010; 74: 306-312.
- Almeida OP, Garrido GJ, Beer C, et al. Cognitive and brain changes associated with ischaemic heart disease and heart failure. Eur Heart J. 2012; 33: 1769-1776.
- Ovsenik A, Podbregar M, Fabjan A. Cerebral blood flow impairment and cognitive decline in heart failure. Brain Behav. 2021; 11: e02176.
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity 2012; 2: 125-141.
- Park B, Roy B, Woo MA, et al. Lateralized resting-state functional brain network organization changes in heart failure. PLoS One. 2016; 11: e0155894.
- Iadecola C. The neurovascular unit coming of age: A journey through neurovascular coupling in health and disease. Neuron. 2017; 96: 17-42.
- Alves TC, Rays J, Fraguas Jr R, et al. Localized cerebral blood flow reductions in patients with heart failure: A study using 99mTc-HMPAO SPECT. J Neuroimaging. 2005; 15: 150-156.
- Yoshiura T, Hiwatashi A, Yamashita K, et al. Simultaneous measurement of arterial transit time, arterial blood volume, and cerebral blood flow using arterial spin-labeling in patients with Alzheimer disease. AJNR Am J Neuroradiol. 2009; 30: 1388-1393.
- Love S, Miners JS. Cerebrovascular disease in ageing and Alzheimer's disease. Acta Neuropathol. 2016; 131: 645-658.
- Miners JS, Palmer JC, Love S. Pathophysiology of hypoperfusion of the precuneus in early Alzheimer's disease. Brain Pathol. 2016; 26: 533-541.
- Schroeter ML, Stein T, Maslowski N, Neumann J. Neural correlates of Alzheimer's disease and mild cognitive impairment: A systematic and quantitative meta-analysis involving 1351 patients. NeuroImage. 2009; 47: 1196-1206.
Figures
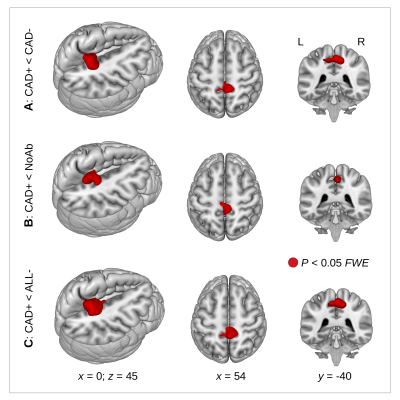
Figure 1. A decrease in network centrality (global correlation, GCOR, red color) was observed in coronary artery disease (CAD) patients with heart failure (CAD+). This GCOR decrease was obtained (A) when comparing CAD+ patients with CAD patients without heart failure (CAD-), (B) when comparing CAD+ patients with patients where no abnormality was detected (NoAb), and (C) when comparing CAD+ patients with all participants showing no heart failure (ALL-). P<0.05 using FWE correction at cluster-level.
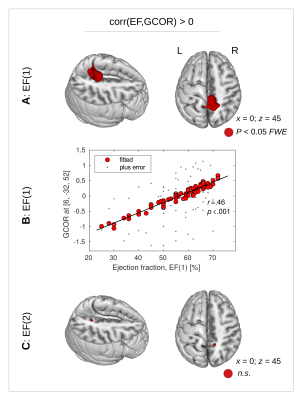
Figure 2. (A) A significant positive correlation was detected between the initial measurement of ejection fraction (EF(1)) and global correlation (GCOR) in the precuneus (red color, P<0.05 with FWE-correction at clusterlevel). (B) Dot-plot showing reduced EF(1) values with diminished GCOR. (C) The follow-up measurement EF(2) did not show any significant correlation with GCOR, neither positive nor negative. n.s. – not significant.
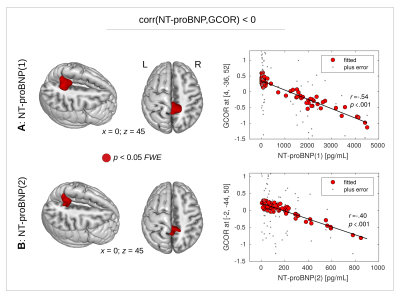
Figure 3. A significant negative correlation was also found between N-terminal fragment of brain natriuretic peptide (NT-proBNP) and global correlation (GCOR) in the precuneus (red color, P<0.05 with FWE-correction at cluster-level). This correlation was obtained (A) with the initial measurement NT-proBNP(1), and (B) with the follow-up measurement NT-proBNP(2). Dot-plots show the correlation using the fitted GCOR values within the statistical model while smaller dots show the zero-mean GCOR values.
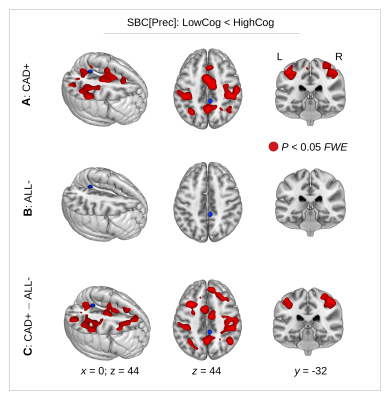
Figure 4. (A) In patients with coronary artery disease and heart failure (CAD+), a seed-based connectivity (SBC) analysis showed reduced precuneus connectivity in patients with lower (LowCog) cognitive performance. (B) No SBC differences were found in patients without heart failure (ALL-). (C) A significant interaction was obtained between the factors “heart failure” (CAD+/ALL-) and “cognition” (LowCog/HighCog) showing diminished SBC with LowCog in heart failure. P<0.05 using FWE correction at cluster-level.
DOI: https://doi.org/10.58530/2022/2050