2049
Combined microstuctural and sodium homeostasis alterations in ALS are widespread in fast progressors: a brain DTI and sodium MRI study1Aix Marseille Univ, CNRS, CRMBM, Marseille, France, 2APHM, Hopital de la Timone, CEMEREM, Marseille, France, 3APHM, Hôpital de la Timone, Referral Centre for Neuromuscular Diseases and ALS, Marseille, France
Synopsis
Amyotrophic lateral sclerosis (ALS) is a heterogeneous condition showing variable progression rates. Conventional MRI lacks sensitivity and specificity to detect abnormalities in ALS and is mainly used to exclude ALS-mimics. Non-conventional MRI has gradually characterized specific neurodegeneration features in ALS.The present brain DTI and sodium MRI study evidenced combined microstructural and sodium homeostasis alterations in ALS and widespread damage in fast progressors. Our results confirm previous reports showing that non-conventional MRI technics might contribute to the diagnostic work-up of patients with different clinical profiles, especially when combined with mathematical modeling enabling predicting disease progression trajectory of a single patient.
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a relentlessly progressive neurodegenerative disorder leading to paralysis and ultimately death within 3 years. ALS is a heterogeneous condition showing variable progression rates. Conventional MRI lacks sensitivity and specificity to detect abnormalities in ALS and is mainly used to exclude ALS-mimics. Non-conventional MRI has gradually characterized specific neurodegeneration features in ALS1. Notably, diffusion MRI showed variable microstructural alterations according to disease progression rate2-3. More recently, a sodium MRI study in ALS showed increase of total sodium concentration (TSC) reflecting brain ionic homeostasis disturbance4.We aimed at investigating the topography of brain regions showing a combined microstructural and sodium homeostasis alterations in ALS subgroups according to their disease progression rates.METHODS
SubjectsPatients with ALS (n=29, mean age 54±10 years, mean disease duration (DD) 1.6±1.2 years) and age and gender-matched healthy controls (HC) (n=24, mean age 51±11 years) were recruited. Patients were clinically scored on the revised ALS Functional Rating Scale (ALSFRS-R)5,6. Patients were clinically differentiated in fast and slow progressors according to their ALFSRS-R rate of progression (i.e. [48-ALSFRS-R]/DD in months; threshold = -0.5 per month).
MRI acquisition
MRI acquisition was performed on a 3T Verio system (Siemens, Erlangen, Germany) using a 23Na-1H head coil (Rapid Biomedical, Rimpar, Germany) and a 32-channel phased-array 1H head coil.
1H-MRI protocol included a T1-weighted (T1w) 3D-Magnetization-Prepared Rapid Acquisition Gradient-Echo (MPRAGE) sequence (TE/TR/TI=3/2300/0.9 ms, 160 slices, resolution=1 mm isotropic, TA=6 min), and a diffusion EPI sequence (64 encoding directions, b=1000 s/mm2 and a b0, TE/TR=95/10700 ms, 60 contiguous slices, resolution=2 mm isotropic, TA=12 min).
23Na MRI protocol included a 3D density-adapted radial sequence (TE/TR=0.2/120 ms, 17000 projections; resolution=3.6 mm isotropic, TA=34 min) and two reference tubes of 50 mmol/L of sodium placed within the FOV for quantification4,7,8.
Data processing
Anatomical. T1w images were normalized to the Montreal National Institute 152 template standard space (MNI152)9.
Diffusion tensor imaging. Diffusion images were denoised and corrected for eddy currents and head motion10,11. Fractional anisotropy (FA), mean diffusivity (MD) maps were computed by fitting a tensor model12. Normalized and skeletonized FA and MD images were generated using TBSS-FSL tools, prior to voxel-wise analysis12,13.
Sodium imaging. Sodium images were reconstructed offline, denoised, and then normalized relative to reference tubes signal to compute quantitative TSC4,8. Spatially normalized TSC maps (MNI152) were smoothed with a Gaussian kernel (8 × 8 × 8 mm), prior to voxel-wise analysis12.
Statistical analysis
Groups comparisons. Differences for age, disease duration, gender and the ALSFRS-R score between groups were assessed using Student t-test, Kruskal-Wallis test and Chi-square test, when applies.
Voxel-wise analysis. Differences in TSC and DTI maps between groups were assessed using permutation inference statistics (5000 permutations), combined with t-testing. Threshold-free cluster enhancement (TFCE) with a significance interval of p-value < 0.05 was used to correct for multiple comparisons12.
RESULTS
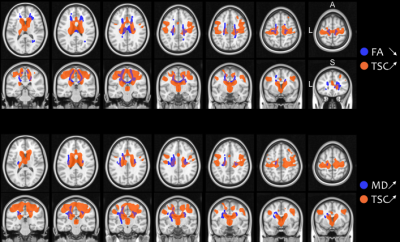
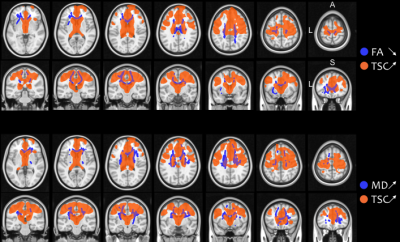
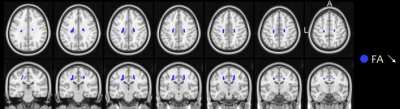
There were no significant differences in demographical nor in clinical data between groups (all p-values > 0.05), except for disease progression rate between fast and slow progressors (p < 0.001) (Table 1).Both ALS patients and fast progressors showed significantly higher TSC and lower FA, higher TSC and higher MD, compared to HC mainly at the level of the body and the genu of corpus callosum, the corticospinal tracts and the corona radiata (Figure 1). Fast progressors showed wider spread abnormalities than in the whole ALS group, including additional tracts such as the forceps minor, the longitudinal fasciculus and the thalamic radiation as well as the frontal pole as demonstrated by TSC (Figure 2). In slow progressors, only FA showed abnormalities, located in focal regions of the corticospinal tracts, the body of corpus callosum, the corona radiata and the thalamic radiation, when compared to HC (Figure 3). There were no significant differences in TSC, neither in DTI between fast and slow progressors.
DISCUSSION
This study highlighted brain regions with common microstructural and sodium homeostasis disturbances corresponding to relevant regions involved in ALS. Interestingly, fast progressors showed widespread combined alterations while slow progressors only showed restricted microstructure damage. Recently, increasing evidences emphasized that heterogeneous disease progression rates influence diagnosis, prognosis and might affect the responsiveness to treatment. Therefore, there is an urgent need of patient stratification. Our results confirm previous reports showing that non-conventional and multiparametric MRI technics might contribute to the diagnostic work-up of patients with different clinical profiles1, especially when combined with mathematical modeling and artificial intelligence to predict disease progression trajectory of a single patient14.CONCLUSION
The present brain DTI and sodium MRI study evidenced combined microstuctural and sodium homeostasis alterations in ALS and widespread damage in fast progressors patients.Acknowledgements
We are grateful to all subjects and their relatives. This research was funded by APHM (AORC Junior 2014 program), ARSLA (Association pour la Recherche sur la Sclérose Latérale Amyotrophique et autres maladies du motoneurone) and FRC (Fédération pour la Recherche sur le Cerveau).References
1- Kassubek J, Müller HP. Advanced neuroimaging approaches in amyotrophic lateral sclerosis: refining the clinical diagnosis. Expert Rev Neurother. 2020 Mar;20(3):237-249.
2- Steinbach R, Gaur N, Roediger A, et al. Disease aggressiveness signatures of amyotrophic lateral sclerosis in white matter tracts revealed by the D50 disease progression model. Hum Brain Mapp. 2021 Feb 15;42(3):737-752.
3- Müller HP, Agosta F, Riva N, et al. Fast progressive lower motor neuron disease is an ALS variant: A two-centre tract of interest-based MRI data analysis. Neuroimage Clin. 2017 Oct 14;17:145-152.
4- Grapperon AM, Ridley B, Verschueren A, et al. Quantitative Brain Sodium MRI Depicts Corticospinal Impairment in Amyotrophic Lateral Sclerosis. Radiology. 2019 Aug;292(2):422-428.
5- Brooks BR, Miller RG, Swash M, Munsat TL; World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000 Dec;1(5):293-9.
6- Cedarbaum JM, Stambler N, Malta E, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci. 1999 Oct 31;169(1-2):13-21.
7- Nagel AM, Laun FB, Weber MA, et al. Sodium MRI using a density-adapted 3D radial acquisition technique. Magn Reson Med. 2009 Dec;62(6):1565-73.
8- Ridley B, Marchi A, Wirsich J, et al. Brain sodium MRI in human epilepsy: Disturbances of ionic homeostasis reflect the organization of pathological regions. Neuroimage. 2017 Aug 15;157:173-183.
9- Avants BB, Tustison NJ, Stauffer M, et al. The Insight ToolKit image registration framework. Front Neuroinform. 2014 Apr 28;8:44.
10- Manjón JV, Coupé P, Concha L, et al. Diffusion weighted image denoising using overcomplete local PCA. PLoS One. 2013;8(9):e73021.
11- Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016 Jan 15;125:1063-1078.
12- Jenkinson M, Beckmann CF, Behrens TE, et al. FSL. Neuroimage. 2012 Aug 15;62(2):782-90.
13- Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006 Jul 15;31(4):1487-505.
14- Meier JM, van der Burgh HK, Nitert AD, et al. Connectome-Based Propagation Model in Amyotrophic Lateral Sclerosis. Ann Neurol. 2020 May;87(5):725-738.
Figures