2045
Increased liver stiffness and shortened T2* relaxation time in people with type 2 diabetes1Institute for Clinical Diabetology, German Diabetes Center, Leibniz Institute for Diabetes Research at Heinrich Heine University, Düsseldorf, Germany, 2German Center for Diabetes Research (DZD e.V.), München-Neuherberg, Germany, 3Institute for Biometrics and Epidemiology, German Diabetes Center, Leibniz Institute for Diabetes Research at Heinrich Heine University, Düsseldorf, Germany, 4Department of Radiology and Nuclear Medicine, Maastricht University Medical Center, Maastricht, Netherlands, 5Department of Endocrinology and Diabetology, Medical Faculty and University Hospital, Heinrich Heine University, Düsseldorf, Germany
Synopsis
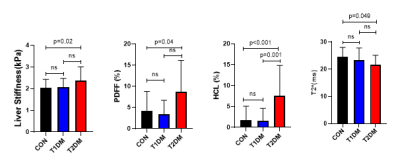
This study shows the value of multiparametric magnetic resonance measurements for detecting the changes in liver tissue due to diabetes mellitus. Liver fat content (determined by mDixon MRI and 1H-MR spectroscopy), T2* relaxation time and liver stiffness were compared in humans with type 1 (T1DM), type 2 diabetes mellitus (T2DM) or normal glucose tolerance (control, CON).
Introduction
Noninvasive magnetic resonance (MR) methods provide valuable information about the structure and function of liver tissue and metabolism, which helped to gain novel insights into pathophysiology of diabetes. Recent studies already show associations of insulin resistance not only with hepatocellular lipid contents (HCL) but also with biomarkers of liver fibrosis (1). However, our understanding of changes in liver metabolism in diabetes is still limited, as studies using multiparametric imaging combining spectroscopy, imaging, and elastography measurements are scarce. Hepatic steatosis can be assessed by MR spectroscopy and imaging (2); liver iron content is commonly assessed with MR methods based on determination of T2* relaxation time (3). Liver stiffness measurement obtained from magnetic resonance elastography (MRE) is the most accurate biomarker for noninvasive liver fibrosis evaluation (4). Thus, the aim of this study was to perform multiparametric MR for simultaneous detection of changes in liver occurred in overt diabetes mellitus.Methods
After consenting to the protocol, approved by the local institutional review board, a total of 144 patients (52 T1DM (age: 39.9±12.1 years; body mass index (BMI): 25.1±4.0 kg/m²), 42 T2DM (age: 57.4±10.6 years; BMI: 28.7±4.6 kg/m²), and 50 healthy controls (age: 44.2±14.7 years; BMI: 26.3±4.9 kg/m²) underwent multiparametric liver MR measurements, combining MR spectroscopy, imaging, and elastography. All MR measurements were performed on a 3T MR scanner (Achieva dStream X-series, Philips Healthcare). All acquisitions were conducted with participants performing a breath-hold. Liver proton density fat fraction (PDFF) and T2*maps were determined from mDixon-Quant measurements, i.e. 6-echo 3D Dixon sequence with the following parameters: TR = shortest possible, TE = 1, 1.7, 2.4, 3.1, 3.8, 4.5 ms; flip angle = 3º, slice thickness = 3 mm with no slice gap. PDFF and T2* values were calculated by placing four regions of interest (ROIs) on the corresponding axial 6-point Dixon images at the portal vein level (5). Liver stiffness was measured using a 2D gradient-echo – MRE sequence with the following parameters: TR = 50 ms, TE = 20 ms, motion encoding gradient frequency = 60 Hz. Four slices of 10 mm thickness with 1 mm gap were acquired in axial plane at the largest portion of the liver in coronal view, avoiding the heart, the liver dome, and the liver bottom tip. An MR elastogram was generated by using the MREview package (Philips, Best, Netherlands). Single voxel STEAM 1H-MRS was performed for quantitative assessment of HCL (TR/TE=4000/10 ms). Both water suppressed and non-suppressed 1H-MRS was performed in the identical voxel within a homogeneous part of liver tissue, avoiding major vessels and gallbladder, with a volume of interest (VOI) size of 25x25x25 mm3. Liver spectra were processed using jMRUI software. HCL content (%) was calculated by the methylene peak at 1.3 ppm in water-suppressed MRS, relative to the sum of the methylene and water peaks at 4.7 ppm in water non-suppressed MRS. Statistical analysis for T1DM, T2DM, and controls was performed using ANOVA with generalized linear model (GLM), adjusted for age, sex and BMI. Tukey’s test was performed for multiple comparison procedure. P values <0.05 were considered to indicate statistically significant differences. Statistical analysis was performed with SAS (version 9.3; SAS Institute, Cary, NC).Results
People with T2DM had higher PDFF as well as HCL, lower T2* values, and higher liver stiffness than controls (Figure 1). In comparison to the T1DM group, liver fat content was higher by MR spectroscopy (HCL), but not with PDFF (p=0.06). Liver stiffness and T2* values also were not different between T1DM and T2DM groups. No differences for any of the measured values were found for T1DM in comparison to healthy humans.Discussion and Conclusion
Spectroscopy-based hepatic fat content (HCL) was more sensitive to detect the differences in liver fat content between the T1DM and T2DM group, which may be due to the known overestimation of hepatic lipid content using mDixon for fat fractions less than 5%. Interestingly, although liver stiffness was in the normal range for all three groups, values were statistically elevated in T2DM, indicating increased fibrosis in this group of patients. T2* is generally used to quantify hepatic iron content and a shorter T2*, as found for the T2DM group, is usually interpreted as an increased iron content. However, as hepatic fat content may also influence T2*, the current data needs to be interpreted with care and future phantom studies will need to address potential lipid-based confounding of T2* as iron indicator.Acknowledgements
No acknowledgement found.References
[1] Zaharia OP, Strassburger K, Strom A, et al. Risk of diabetes-associated diseases in subgroups of patients with recent-onset diabetes: a 5-year follow-up study. Lancet Diabetes Endocrinol 2019;7:684-694.
[2] Kukuk GM, Hittatiya K, Sprinkart AM, et al. Comparison between modified Dixon MRI techniques, MR spectroscopic relaxometry, and different histologic quantification methods in the assessment of hepatic steatosis. Eur Radiol. 2015;25:2869-79.
[3] Labranche R, Gilbert G, Cerny M, et al. Liver Iron Quantification with MR Imaging: A Primer for Radiologists. Radiographics. 2018;38(2):392-412.
[4] Xiao G, Zhu S, Xiao X, et al. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fbrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology 2017;66:1486–1501.
[5] Campo CA, Hernando D, Schubert T, et al. Standardized Approach for ROI-Based Measurements of Proton Density Fat Fraction and R2* in the Liver. AJR Am J Roentgenol. 2017;209(3):592-603.