2042
Systemic metabolic dysregulation common across multiple organscaused by cancer and cancer-induced cachexia
1Division of Cancer Imaging Research, The Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2Sidney Kimmel Comprehensive Cancer Center, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3Radiation Oncology and Molecular Radiation Sciences, The Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
Analysis of organ metabolism with cancer growth can unravel systemic changes that occur with cancer and with cancer-induced cachexia. Cancer-induced cachexia, commonly observed with pancreatic cancer, causes tissue wasting, intolerance to chemotherapy, and poor quality of life. Here, we identified alterations of metabolic pathways in the spleen, liver, pancreas, lung, heart, brain and kidney following growth of cachexia-inducing and non-cachexia inducing pancreatic cancer xenografts. These results highlight the systemic metabolic changes that occur with cancer and with cancer induced cachexia that may lead to the development of organ-based early biomarkers as well to organ-based metabolic treatment strategies.
Introduction
Cancer induced cachexia is a multifactorial syndrome occurring with high frequency in pancreatic cancer that results in unexplained weight loss in cancer patients. Cachexia results in morbidity and mortality, a low tolerance to chemotherapy, and overall treatment failure [1,2,3]. Cachectic patients experience a wide range of symptoms affecting the function of organs, such as the liver, brain, and heart, causing significant morbidity [4]. Cachexia induced metabolic changes in body organs have not been previously investigated. Here, for the first time, we have characterized common metabolic pathways in the spleen, liver, pancreas, lung, brain, heart, and kidney to understand the systemic metabolic dysregulation caused by cancer and by cancer-induced cachexia.Method
The non-cachexia inducing human pancreatic cancer cell line, Panc1, was established from a primary pancreatic ductal adenocarcinoma from a 56-year-old male patient and was obtained from ATCC. The cachexia inducing human pancreatic cancer cell line, Pa04C, provided by Dr. Maitra at the Johns Hopkins University School of Medicine, was isolated from a lung metastasis in a 59-year-old male patient with stage IV pancreatic adenocarcinoma[5]. Six- to eight- week- old severe combined immunodeficient (SCID) male mice were used in these studies. Panc1 or Pa04C cells (2 × 106 cells) in 50 μL of Hanks solution were injected into the right flank of mice. Control, cachectic and non-cachectic groups consisted of 10, 10 and 9 mice per group respectively. Mice with comparable Pa04C and Panc1 tumor volumes were sacrificed once tumors were ~ 500 mm3. Organs from tumor bearing mice, and normal mice of similar age, were harvested, freeze clamped and stored at -80°C for 1H MRS analysis.Snap frozen organs were pulverized under liquid nitrogen, weighed, and dual phase extraction was performed, as described previously [6]. The aqueous phase was collected, evaporated under a stream of nitrogen, and lyophilized to remove the remaining water. Samples were reconstituted in 650 μl of 1x phosphate buffered D2O (90% D2O, 10% H2O, pH = 7.4) containing trimethylsilylpropionic acid (TSP), vortexed, centrifuged at 500g for 5 min at 4o C, and supernatants were subjected to 1H MRS analysis. Briefly, all 1H MR spectra were acquired at room temperature on a Bruker Avance III 750 MHz (17.6 T) MR spectrometer equipped with a 5 mm probe. Areas under the peaks were integrated and normalized with respect to TSP as well as to the tissue weights used for dual phase extraction. Quantitative analyses of small molecules like amino acids, organic acids, choline compounds, glucose, lactate were performed with the aqueous phase extracts of all organs. Statistical analysis was performed using an unpaired one-tailed student t-test. Metabolites that showed significant differences were subjected to pathway impact analysis using MetaboAnalyst 5.0 [7]
Result and Discussion
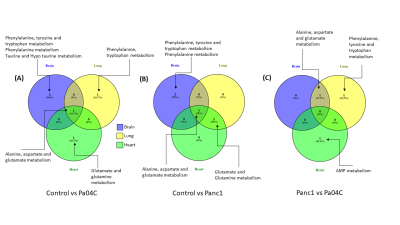
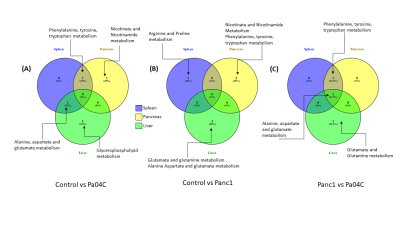
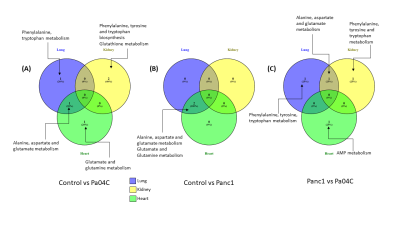
Cachexia-inducing Pa04C tumors induced significant weight loss in mice compared to Panc1 tumor bearing mice or normal mice as previously observed [8]. Weights of all the organs were significantly lower in cachexia inducing Pa04C tumor bearing mice compared to normal control mice. Metabolites that showed significant changes (p<0.05) in comparisons of control vs Panc1, control vs Pa04C and Panc1 vs Pa04C were analysed for pathways impact analysis using MetaboAnalyst to investigate the change in associated metabolic pathways. Multiple separate Venn diagrams (Figures 1-3) were created to summarize metabolic changes and their commonality in organs from normal mice compared to Pa04C tumor bearing cachectic and Panc1 tumor bearing non-cachectic mice. Organ-specific changes in metabolic pathways were identified in all the organs investigated, including highly critical organs such as the heart and brain, highlighting the systemic metabolic reprogramming that occurs with tumor growth and with cachexia. Alanine, aspartate and glutamate metabolism was commonly altered across the brain, heart and lung in control vs Pa04C mice and control vs Panc1 mice, and in the brain and lung in Panc1 vs Pa04C mice, (Fig. 1). Changes in phenylalanine, tyrosine and tryptophan metabolism were common in the spleen, pancreas and liver in control vs Pa04C mice and between Panc1 mice vs Pa04C mice; alanine, aspartate and glutamate metabolism changes were common in the spleen, pancreas and liver in Panc1 vs Pa04C mice (Fig.2). The lung and heart showed changes in alanine, aspartate and glutamate metabolism in control vs Pa04C mice, and control vs Panc1 mice but these metabolic pathway changes were not common to the kidney (Fig. 3).These results highlight the systemic changes in metabolic pathways that occur with pancreatic cancer and with pancreatic cancer induced cachexia that may lead to the development of early biomarkers based on alterations of metabolic pathways in organs, as well to metabolic treatment strategies to reduce morbidity and mortality from pancreatic cancer.
Acknowledgements
Acknowledgement: This work was supported by NIH R35CA209960 and R01CA193365.References
1. Argiles JM et al., Nature reviews Cancer, 2014.
2. Dore-Savard L et al., Scientific Reports, 2016.
3. Fearon KC et al., HPB (Oxford), 2010.
4. Inui A et al., CA Cancer J Clin, 2002.
5. Ozola Zalite I et al., Pancreatology, 2015.
6. Winnard Jr PT et al., Journal of Cachexia, Sarcopenia and Muscle, 2020.
7. Pang Z et al., Nucleic Acids Research, 2021.
8. Winnard PT, Jr. et al., Cancer Res: 2016.
Figures