2037
Distortion Correction in Abdominal DW-MRI using Dual-Echo EPI for Improved Evaluation of Crohn’s Disease1Radiology, Boston Children's Hospital and Harvard Medical School, Boston, MA, United States
Synopsis
In MR enterography, diffusion-weighted MRI (DW-MRI) is commonly used to evaluate Crohn’s disease (CD). Echo-planar imaging (EPI) used in DW-MRI suffers from geometric distortion due to time-varying susceptibility field changes in the bowel. Hence, we implemented a DW-MRI sequence using a dual-echo EPI with opposing encoding polarities. Thus, the distortion field can be estimated dynamically and corrected retrospectively. The proposed method was evaluated in DW-MRI data acquired on suspected CD patients by visually and quantitatively assessing distortion corrected volumes based on anatomical similarity with T2-HASTE and uncertainty of estimated diffusion parameters of the intravoxel incoherent motion model.
Introduction
MR enterography is frequently used to assess disease activity for Crohn’s disease (CD)1. Diffusion-weighted MR imaging (DW-MRI) can be used to identify bowel segments with active inflammation. Because of their speed, echo-planar imaging (EPI) sequences are commonly used for DW-MRI. A common drawback of EPI-based MRI sequences is the degradation of image quality due to geometric distortions caused by field inhomogeneity, gradient nonlinearity, eddy currents, and susceptibility differences in the imaged tissues. Due to high susceptibility differences in the bowel, geometric distortions can be severe in DW-MRI especially in 3T MRI scanners. In conventional distortion correction techniques, DW images are acquired with opposing encoding polarities and the estimated distortion field is used for correcting all b-value images. However due to motion in the bowel, the distortion field changes during the acquisition of multiple images. We, therefore, acquire DW images using a dual-echo EPI sequence with opposing encoding polarities acquired at echo one and two respectively2,3. These echos are acquired only 40ms apart, effectively freezing motion. Thus, the distortion field can be estimated dynamically from these two echos and corrected retrospectively. We evaluated our method in DW-MRI data acquired on suspected CD patients by evaluating applied distortion correction based on anatomical similarity with T2-HASTE images and uncertainty of estimated parameters of the intravoxel incoherent motion (IVIM) model4.Methods
A dual-echo EPI sequence is implemented and abdominal diffusion-weighted MR images were included in the clinical protocol of 7 patients (5 female, 2 male, age=16.1±2.9 years) who had suspected CD after informed consent according to the IRB protocol. Two echos were acquired with blip-reversed phase encodings (i.e., A→P and P→A) for each of the 51 DW-MR volumes (8 b-values and 6 directions) using a 3T Siemens Prisma/Skyra scanner (Siemens Healthcare, Erlangen, Germany). We acquired 60 axial slices with the imaging parameters: TE1/TE2=58ms/98ms, TR=8300ms FOV=360x270mm2 in-plane resolution of 1.1x1.1mm2 with 5mm slice thickness; b-values = 0, 20, 50, 100, 200, 400, 600, 800. Scaled FSL topup5 was applied to correct for the distortion using two paired echos. An axial T2-HASTE was acquired as part of the clinical protocol. T2-HASTE volumes were matched to b=0 DW-MR volumes by employing a rigid registration to compute dice scores between segmented bowel regions on each. For four subjects, the kidney was segmented instead of the bowel due to the non-rigid motion of the bowel between two acquisitions. Then, a bi-exponential IVIM model was fitted to the segmented ROIs. To assess the uncertainty of fitted IVIM parameters, coefficient of variation (CoV) percentage was calculated over 400 wild-bootstrap repetitions by resampling multiple sets of the diffusion signal for each b-value from the estimated signal model using the bootstrap resampling strategy6.Results
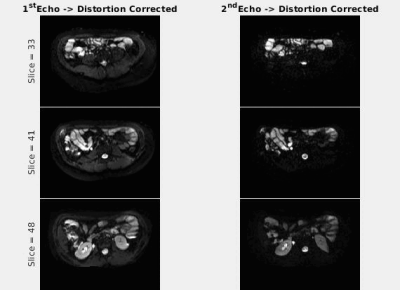
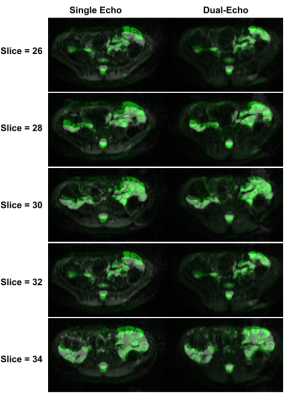
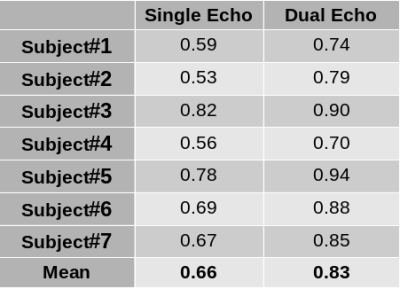
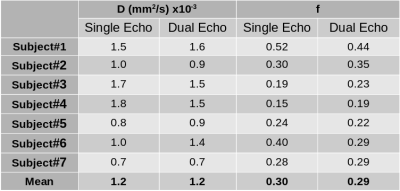
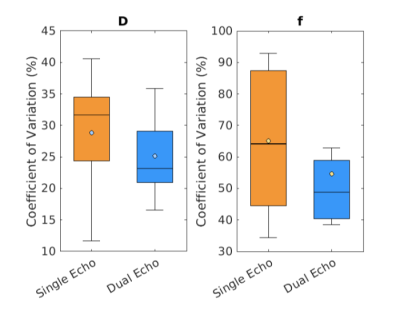
Animated Figure 1 demonstrates the evolution of distortion correction on the first and second echos. Overlay of T2-HASTE on single-echo and distortion corrected DW-MR slices are depicted in Figure 2, for visualization of anatomical similarity of images. Dual echo distortion corrected image is better aligned with T2-HASTE. Dice scores, evaluating the anatomical similarity between T2-HASTE and DW images for single echo and distortion corrected volumes, are given in Table 1 for all subjects. Using distortion corrected dual-echo DW-MRI, dice scores were improved from 0.66 to 0.83. Estimated D (slow diffusion) and f (perfusion fraction) values for single echo and distortion corrected volumes are shown in Table 2. Box-plots of coefficient variation percentages are given in Figure 3.Discussion and Conclusion
Geometric distortions are reduced by applying FSL topup on the dual-echo volumes (Figure 1), resulting in improved similarity to anatomical scan (T2-HASTE) as observed visually in Figure 2. Furthermore, improved dice scores quantitatively verified improved anatomical matching due to distortion correction (Table 1). In Table 2, estimated D (slow diffusion) and f (perfusion fraction) values are consistent with the ones reported in the literature7,8, indicating some patients have restricted diffusion in their bowels due to suspected CD. For D value, median CoV percentages are reduced from 32.6% to 23.2% when using distortion corrected dual-echo. For f value, median CoV percentages were reduced from 64.1% to 48.7% when using distortion corrected dual-echo compared to single-echo (Figure 3).As the bowel motion is non-rigid, acquiring separate AP/PA scans does not effectively solve the distortion problem. This is because the two images will have different bowel shapes due to motion between the respective AP and PA scans. Our results show that the proposed distortion correction method can effectively correct time-varying geometric distortions and misalignments and improve the precision of the quantitative parameters of the IVIM model.
Acknowledgements
This work was supported partially by the Society of Pediatric Radiology Multicenter Research Grant 2019, by the National Institutes of Health under award numbers R01EB019483, R21DK123569, R21EB029627, 1R01DK125561-01A1, and by the grant No 2019056 from the United States- Israel Binational Science Foundation (BSF), and a pilot grant from National Multiple Sclerosis Society under Award Number PP-1905-34002.References
1. Bruining DH, Zimmermann EM, Loftus EV, Sandborn WJ, Sauer CG, Strong SA. Consensus recommendations for evaluation, interpretation, and utilization of computed tomography and magnetic resonance enterography in patients with small bowel Crohn’s disease. Gastroenterology 2018;154:1172–1194.
2. Afacan, O., Hoge, W.S., Wallace, T.E., Gholipour, A., Kurugol, S. and Warfield, S.K., 2020. Simultaneous Motion and Distortion Correction Using Dual‐Echo Diffusion‐Weighted MRI. Journal of Neuroimaging, 30(3), pp.276-285
3. Coll‐Font, Jaume, et al. "Retrospective Distortion and Motion Correction for Free‐Breathing DW‐MRI of the Kidneys Using Dual‐Echo EPI and Slice‐to‐Volume Registration." Journal of Magnetic Resonance Imaging 53.5 (2021): 1432-1443.
4. Le Bihan, D. , Breton, E. , Lallemand, D. , Aubin, M.L. , Vignaud, J. , Laval-Jeantet, M. , 1988. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168 (2), 497–505.
5. Andersson JL, Skare S, and Ashburner J, How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 2003;20:870–88.
6. Freiman M, Voss SD, Mulkern RV, Perez-Rossello JM, Warfield SK. Medical Image Computing and Computer-Assisted Intervention–MICCAI 2011. Springer; 2011. Quantitative body DW-MRI biomarkers uncertainty estimation using unscented wild-bootstrap; pp. 74–81.
7. Kurugol, Sila, et al. "Spatially-constrained probability distribution model of incoherent motion (SPIM) in diffusion weighted MRI signals of crohn’s disease." International MICCAI Workshop on Computational and Clinical Challenges in Abdominal Imaging. Springer, Cham, 2014.
8. Zhang, Meng-Chen, et al. "IVIM with fractional perfusion as a novel biomarker for detecting and grading intestinal fibrosis in Crohn’s disease." European radiology 29.6 (2019): 3069-3078.
Figures