2034
Machine learning-based texture analysis of IVIM-DKI for classification of benign and malignant pancreatic masses1Centre for Biomedical Engineering, Indian Institute of Technology Delhi, New Delhi, India, 2Department of Radiodiagnosis, All India Institute of Medical Sciences Delhi, New Delhi, India, 3Department of Gastroenterology, All India Institute of Medical Sciences Delhi, New Delhi, India, 4Department of Pathology, All India Institute of Medical Sciences Delhi, New Delhi, India, 5Department of Biomedical Engineering, All India Institute of Medical Sciences Delhi, New Delhi, India
Synopsis
Tumor heterogeneity could be detected non-invasively utilizing textural indicators from IVIM-DKI, which have a high potential for early prognosis of pancreatic lesions. A novel technique was investigated for tumor prediction model utilizing IVIM-DKI with total variation penalty function, in which we employed combinations of texture characteristics from IVIM-DKI parameters. In this study, texture characteristics of the kurtosis(k) parameter had the high accuracy:93% and AUC:1, and combinations of all IVIM-DKI parameters' textural features after feature reduction had accuracy:84% and AUC:0.91 for classifying benign and malignant pancreatic lesions. Whole-volumetric texture analysis of IVIM-DKI can be employed for characterization of pancreatic lesions.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) accounts for over 90% of malignant neoplasm of the pancreas1. The clinical manifestations are non-specific which leads to a delay in diagnosis and consequently poor prognosis. Therefore, it is necessary to identify biomarkers for early detection of pancreatic cancers. Image-guided fine-needle aspiration cytology (FNAC) currently offers reliable findings for characterization of pancreatic lesions; however, it is susceptible to tumor seeding in the sampling tract and sampling errors2. Texture analysis (TA) on MRI can non-invasively characterize pancreatic lesions and help in planning management3. No prior studies have evaluated the TA of parameters estimated using intravoxel incoherent motion coupled with diffusion kurtosis imaging (IVIM-DKI) in classifying benign and malignant pancreatic lesions. This study explores the application of a novel method in IVIM-DKI signal modeling with integration of the total variation (TV) penalty function to reliably estimate the IVIM-DKI parameters4,5. In addition, to study the effect of TA on IVIM-DKI parameters using this novel model in the characterization of benign and malignant pancreatic lesions.Methods
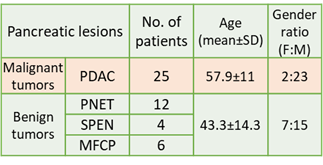
Study population: Based on the FNAC/biopsy or MRI findings, total of 47 patients with pancreatic lesions were divided into benign tumor (n=22;mean±SD=43.3±14.3years;F:M=7:15) comprising of solid pseudopapillary epithelial neoplasm (SPEN:n=4), mass-forming chronic pancreatitis (MFCP:n=6), and pancreatic neuroendocrine tumor (PNET:n=12) and malignant tumors included only pancreatic ductal adenocarcinoma (n=25; mean±SD=57.5±10.9 years; F:M=2:23) as shown in figure 1.IVIM-DKI image analysis: IVIM-DKI sequence was acquired at 1.5T scanner with 14 b-values(number of averages) as 0(1),25(1),50(1),75(1),100(1),150(1),200(1),500(2),800(3), 1000(3),1250(4),1500(4),2000(5),2500(7)s/mm2 covering pancreas using free-breathing technique. Non-linear least-squares optimization and parallel computing with an in-house built toolbox were used for ADC estimation, and IVIM-DKI parameters were generated using ivimDKI3Dtvtool_v1_2 application (https://github.com/amitvmehndiratta/IVIM-DKI-MRMP 2021)5 developed in MATLAB (version 9.9, The MathWorks, Inc., Natick, MA, USA).
Localization of Region of interest: Using ADC maps and morphological sequences like T2-weighted, contrast-enhanced T1-weighted, and DWI as a reference, lesion ROIs were manually drawn throughout the whole volume of mass and delineated in matching slices of IVIM-DKI images.
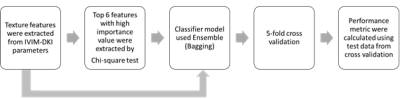
Texture feature extraction and machine learning-based classification: Figure 2 shows the workflow of TA with machine learning-based classification. A total of 30 texture features were extracted from the global texture(3 features), Gray-Level Co-occurrence Matrix(GLCM: 9 features), Gray-Level Run-Length Matrix(GLRLM: 13 features), and Neighborhood Gray-Tone Difference Matrix(NGTDM: 5) with 26-voxel connectivity using toolbox developed by Vallière, M. et al.6. The top six texture features with high importance values were selected using the chi-square test and were used as input to the random forest to classify benign from malignant lesions. Accuracy, precession, recall, specificity, accuracy, F1 score, and classification error on test data were estimated from each fold of 5-fold cross-validation.
Results
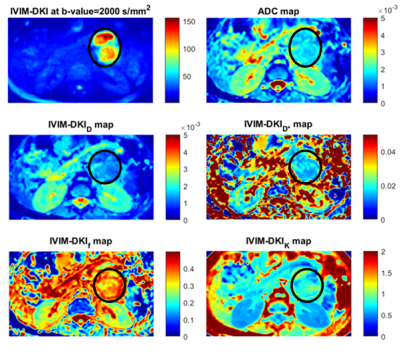
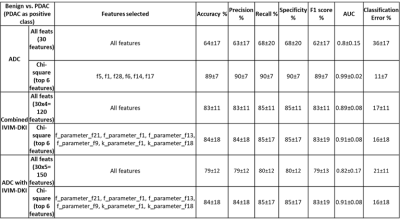
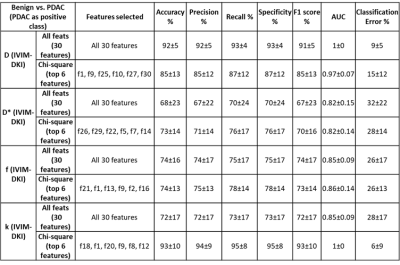
Figure 3 shows parameter maps estimated using novel models that produce improvement in quality of parameter maps. A total of 150 texture features(30*5) were extracted for each lesion using 30 texture features derived from ADC, D, D*, f, and k. All IVIM-DKI parameters and ADC features were combined to form a whole of 120(30 features from each parameter, 30x4) texture feature sets. All features were fed to the classifier model, where texture features from the D(30 textural features from D) were observed to produce a high accuracy:92%, F1 score:91%, and AUC :1 respectively(figure 4). Further, using all IVIM-DKI parameters(120 features) produced equally good results with accuracy and an F1 score of 83%, as shown in figure 4.Both accuracy and F1 score of top six features for k parameter were 93% and AUC of 1 as shown in figure 5. In addition, ADC showed good performance with accuracy and F1 score:89% and AUC:0.99, and similarly, D showed accuracy and F1 score: 85% and AUC:0.97. Except for D, classification performance increased after feature reduction. Figures 4 and 5 illustrate the top six features arrangement in decreasing order based on feature importance value. The top six texture features for both combined IVIM-DKI parameters and ADC with combined IVIM-DKI parameters were found to be the texture features of f and k parameters. Overall, variance, GLRLM texture features such as SRHGE and LGRE have majorly contributed to improvement in classification performance for all combinations except for the parameter D*.
Discussion
Texture analysis can detect non-invasive changes in the intensity of a lesion region and provide information on microscopic tissue heterogeneity that can be utilized to identify pancreatic lesions6. In this study, individual and combination of texture features from IVIM-DKI and ADC with feature reduction by chi-square test were utilized. After feature reduction, the classification performance of k increased significantly in terms of accuracy and AUC. GLCM and GLRLM features of k were selected, these features can capture tissue heterogeneity and complexity, potentially enhancing accurate diagnosis. Due to poor diagnostic performance in PDAC identification, the advantages of kurtosis have not been substantially researched in the literature7. f and k texture features were selected to produce high accuracy and AUC in both combinations of IVIM-DKI parameters and IVIM-DKI with ADC parameters. It can be shown that this combination of IVIM-DKI parameters can offer perfusion and tumor heterogeneity to represent the heterogeneous mass region.Conclusion
Whole-volumetric texture analysis combined with machine learning-based classification of pancreatic masses employing individual or combinations of IVIM-DKI parameters can aid in non-invasive characterization.Acknowledgements
This study was supported by IIT Delhi and AIIMS Delhi. AVM was supported by research fellowship fund from Ministry of Human Resource Development, Government of India.References
- Orth M, Metzger P, Gerum S, et al. Pancreatic ductal adenocarcinoma: biological hallmarks, current status, and future perspectives of combined modality treatment approaches. Radiat Oncol 2019 141 2019; 14:1–20.
- Chang DK, Nguyen NQ, Merrett ND et al. Role of endoscopic ultrasound in pancreatic cancer. Expert Rev Gastroenterol Hepatol. 2014; 3:293–303
- Deng Y, Ming B, Zhou T, et al.: Radiomics Model Based on MR Images to Discriminate Pancreatic Ductal Adenocarcinoma and Mass-Forming Chronic Pancreatitis Lesions. Front Oncol 2021; 11:1.
- Kayal EB, Kandasamy D, Khare K, et al.: Quantitative Analysis of Intravoxel Incoherent Motion (IVIM) Diffusion MRI using Total Variation and Huber Penalty Function. Med Phys 2017; 44:5849–5858.
- Malagi AV, Netaji A, Kumar V, et al.: IVIM–DKI for differentiation between prostate cancer and benign prostatic hyperplasia: comparison of 1.5 T vs. 3 T MRI. Magn Reson Mater Phys Biol Med 2021.
- Vallières M, Kay-Rivest E, Perrin LJ, et al.: Radiomics strategies for risk assessment of tumour failure in head-and-neck cancer. Sci Rep 2017 71 2017; 7:1–14.
- Mayer P, Fritz F, Koell M, et al.: Assessment of tissue perfusion of pancreatic cancer as potential imaging biomarker by means of Intravoxel incoherent motion MRI and CT perfusion: correlation with histological microvessel density as ground truth. Cancer Imaging 2021; 21:1–12.
Figures